Improving Cell Metabolism with Botanical Compounds
Healthy cell metabolism or normal cellular metabolism is when the chemical reactions that occur in living cells are working properly. Our bodies are made up of over 37 trillion human cells: 37,200,000,000,000. For our bodies to work right, our cells must engage in healthy cell metabolism. Plant medicine can be a powerful ally for cancer patients.
Cell metabolism is the set of chemical reactions that occur in living organisms in order to maintain life. Healthy cells that function correctly control their growth. In other words, they only grow and divide into new cells only when necessary, or as instructed by the Life Force – the governing energy that sustains us.
Metabolic Pathways
Cell metabolism involves complex sequences of controlled biochemical reactions, better known as metabolic pathways. These processes allow organisms to grow and reproduce, maintain their structures, and respond to environmental changes. Healthy cells also undergo what is called apoptosis or programmed cell death, as well as autophagy. Autophagy and apoptosis are two important cellular processes with complex and intersecting protein networks.[1] They maintain cellular homeostasis, which is characterized by their morphology and regulated through signal transduction mechanisms.[2] A normal, healthy cell metabolism contains autophagy and apoptosis.
Cancer Cells Can Hijack and Take Control of Metabolic Pathways
Cancer cells, on the other hand, don’t stop growing and dividing. Their uncontrolled cell growth leads to tumors. Cancer cells do not have healthy cell metabolism. They are in fact capable of hijacking or robbing and taking control of metabolic pathways including many of the essential nutrients for healthy cell metabolism. Cancer cells also take control of numerous growth factors and cells including immune cells, such as neutrophils and macrophages, and platelets.[3] Tumors act on a multitude of alternative metabolic mechanisms and are profoundly adaptable. Cancer cells can potently re-wire signaling pathways, modulate survival pathways and even re-structure the cell. [4] In other words, starving cancer cells of specific nutrients, or energy supply, such as with a ketogenic diet, will not provide any sustainable benefit. This is because the cancer cells adapt! A type of tug-of-war occurs between the TUMOR (cancer) and the HOST (individual).
Metabolic Reprogramming
In fact, metabolic reprogramming leads to cancer initiation, progression, and metastasis. The metabolic signature of cancer cells includes alterations in glycolysis, mitochondrial respiration, lipid metabolism, as well as amino acid[5], and mineral metabolism.[6]
More often than not, the microenvironment of solid tumors contains regions of poor oxygenation and high acidity. In this context hypoxia can act in an epigenetic fashion,
inducing changes in gene expression and in metabolism for survival. It is reasonable to assume that only the tumor cells capable of developing an unusual tolerance to limiting oxygen availability and to the acidosis resulting from excessive lactate production, can survive.
For cancer cells to take hold, striking changes occur in glucose metabolism, as I explain in this article. Studies in human cancer patients suggest that there is also an increase in free fatty acid turnover, oxidation, and clearance in these cells. This means that cancer cells are very capable of redirecting metabolic pathways to meet energy demands through the regulation of fatty acid metabolism. This occurs through a lipid mobilizing factor produced by tumor cells that appears to be responsible for the increase in whole-body fatty acid oxidation.[7]
Cancer Cells Need Energy
Healthy cell metabolism involves metabolizing glucose molecules. This is a two-step process. The cell takes the glucose into itself. Then, during the first phase, it begins metabolizing the glucose in the cytoplasm (cytoplasm is most of the cell, everything inside the cell membrane but not in the nucleus.) This process does not involve oxygen. However, the cell also metabolizes the glucose molecules inside the mitochondria. The mitochondria are in the cytoplasm. They are the organelles that produce most of the energy cells need to stay alive.

Phase two of healthy cell metabolism requires oxygen. Cancer cell energy needs are more complex. They use glucose, too, as a source of energy, but they also need more than just glucose.
They can use lactate to fuel their growth and make other compounds they need to continue growing unchecked, according to a 2016 study. Lactate is a partially digested form of glucose. In healthy cell metabolism, it is secreted as a waste product. They also take up amino acids, lysophospholipids, acetate, and extracellular protein to use as fuel.
Although alterations of glycolysis are certainly evident, the metabolic signature of cancer cells also includes alterations in mitochondrial respiration, fatty acid/lipid, and amino acid metabolism.[8]
Ketogenic Diets Not the Answer to Cancer-Disrupted Healthy Cell Metabolism
While some research suggests that a ketogenic diet can help treat cancer by creating an unfavorable metabolic environment for cancer cells, I do not believe it is helpful.
A ketogenic diet is a very low carb high-fat diet. This diet asks adherents to drastically reduce carbohydrates and increase healthy fats in order to put their body in a metabolic state called ketosis.
A ketogenic diet is not a good solution to the problem of cancer cell metabolism, even though, in specific cases, it may be useful for a short period of time. I only restrict complex-whole-food carbs when a patient has ascites (a condition where fluid accumulates in the abdomen) or is struggling with lymphatic drainage. Cancer cells can redirect metabolic pathways to meet energy demands through the regulation of fatty acid metabolism.[9] I wrote more about why a Ketogenic diet is not the best approach to suppressing cancer in this article, “Can A Ketogenic Diet Cure Cancer?”
Alterations of Lipid Metabolism in Cancer cells
Many recent studies have provided significant evidence to support a “lipolytic phenotype” of cancer. Fatty acid oxidation (FAO), like other well-defined metabolic pathways involved in cancer, is dysregulated in diverse human malignancies. Cancer cells rely on FAO for proliferation, survival, stemness, drug resistance, and metastatic progression. FAO is also reprogrammed in cancer-associated immune and other host cells, which may contribute to immune suppression and tumor-promoting microenvironment.[10]
Lipid metabolism and the epigenetic modifying enzymes interact in a bidirectional manner which involves regulating cancer cell death. Within the tumor, microenvironment lipids play unique roles beyond metabolic requirements that promote cancer progression.[11]
Cancer cells, as well as other cell types in the tumor microenvironment, exploit various ways to acquire lipids. They are able to extensively rewire their metabolism as part of a plastic and context-dependent metabolic reprogramming driven by both oncogenic and environmental cues.
The impact of altered lipid metabolism may extend well beyond classical lipid-regulated pathways and affect numerous cellular processes.[12]
Extracellular Vesicles
Cancer cells are also known to release high amounts of extracellular vesicles, which are largely composed of lipids.
This release may help cancer cells to balance their lipid content. They appear to function as critical lipid-based transport vesicles involved in intercellular communication.
Lipid-signaling molecules are released from cells by specific phospholipases, such as
secreted phospholipase A enzymes, and processed to oxidized lipid products or eicosanoids. These lipid-based products are involved in intercellular communication and play an important modulatory role in immune escape and tumor immunology.[13],[14],[15]
Fatty acid synthase (FAS) is the major enzyme required to convert carbohydrates to fatty acids.
Recent evidence suggests that fatty acid synthase FAS activity is essential for cancer growth and survival. Blocking the enzyme activity results in cell death.[16],[17],[18],[19]
Healthy cell metabolism does not involve overexpressing FAS. In fact, tumors overexpressing FAS display aggressive biologic behavior compared to those tumors with normal FAS levels.[20],[21],[22]
Botanical and Dietary Compounds to the Rescue!
Fatty acids, along with mitochondria and glucose metabolism, can be targeted for anticancer treatment using botanical and dietary compounds that then can limit cell proliferation, growth, and transformation.
Emerging concepts utilizing botanical and dietary compounds to induce lipid metabolic reprogramming in cancer that is mainly involved in lipid uptake and trafficking, esterification, fatty acid synthesis and oxidation, lipogenesis, and lipolysis.[23]
Here are some of the top featured compounds that I include in my health-optimizing protocols. These compounds help inhibit cancer growth, in part, through the suppression of fatty acid synthase.
Terpenoids
Terpenoids are a common class of compounds found in plants. Emerging evidence suggests essential roles for terpenoids in alleviating metabolic diseases through their targeting of lipid metabolism. Terpenoids can help your body get back to healthy cell metabolism.
Flavonoids
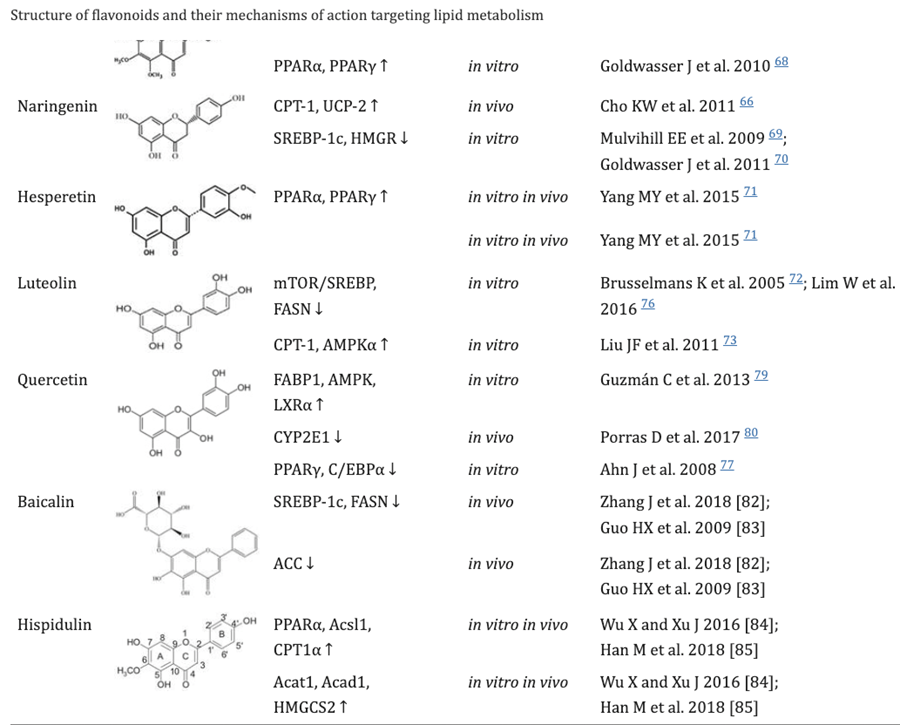
Flavonoids, found in many plants, play an important role in the growth and development of plants as well as disease prevention. They have been shown to ameliorate lipid metabolism. They can be used for the prevention and treatment of metabolic disease and cancer.[25]
Citrus fruits contain various flavonoids, including naringenin, hesperetin, eriodictyol, nobiletin and tangeretin. These are involved in the regulation of lipid metabolism and inhibition of hepatic apoB secretion.[26],[27]
Resveratrol
Resveratrol is a natural polyphenolic compound. It has been shown to exhibit cardio-protective as well as anti-cancer effects on various types of cancers.[28]
Resveratrol also has other, more global, health benefits. I explore these in this article about resveratrol’s unique health-promoting properties.
Catechin-rich Green Tea
Green tea polyphenols, such as catechins, flavones, and theaflavins, are reported to decrease the risk of cancer, mainly by acting on numerous pathways including the pathway for fatty acid synthase.[29],[30]
Catechins are the main bioactive ingredients of green tea, among which epigallocatechin-3-gallate appears to be one of the most powerful antioxidants to prevent damage in cells with healthy cell metabolism as well as providing an anti-tumor effect.
Quercetin
Quercetin is widely found in fruits and vegetables in the human diet. Perhaps my favorite supplement, it has a wide range of health benefits.
Quercetin has several beneficial effects on human health, including:
- cardiovascular protection
- anticancer activity
- anti-inflammatory effects
It can act as an anti-cancer, anti-tumor, anti-ulcer, anti-allergy, anti-viral, anti-inflammation, and anti-diabetes agent, exerting gastro-protection, anti-hypertension, immune-modulation, as well as anti-infection features are among its advantageous effects.[31],[32]
Quercetin is not harmful to healthy cell metabolism. But it can impose cytotoxic effects on cancer cells through several mechanisms.[33]
In addition, quercetin acts as a potent inhibitor of lipogenesis in prostate and breast cancer cells through its inhibition of FAS activity.[34] Hence, quercetin might reduce lipid accumulation and have good therapeutic potential against cancer.[35]
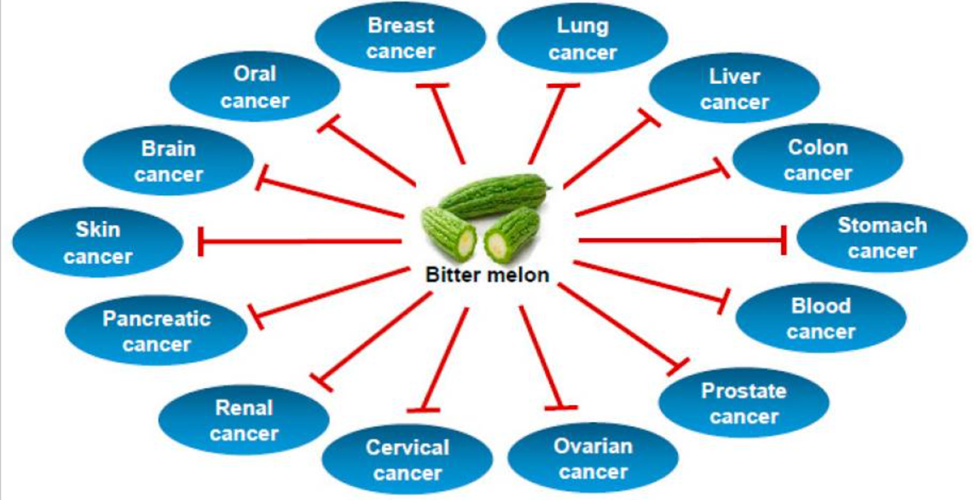
Bitter Melon Extract
Bitter melon (Momordica charantia) is well-known for its hypoglycemic and anti-diabetic effects. It grows tropically in the Amazon, East Africa, Asia, India, South America, and the Caribbean. Bitter melon is used traditionally as both food and medicine.
Bitter melon extract has been shown in several studies to prevent carcinogen-induced cancer in mice. It seems to inhibit glycolysis and lipid metabolism and induce cell death in oral cancer.[36]
Types of Cancer Prevented by Bitter Melon
Dandelion Extract
Dandelion (Taraxacum mongolicum) is a classical herbal medicine used to treat breast diseases based on traditional Chinese medicine as well as in western traditional medicine and was reported to have antitumor effects and lipid regulatory capacities.
Research has shown that dandelions and dandelion extract can inhibits triple-negative breast cancer cell proliferation. It does so by interfering with glycerophospholipids and unsaturated fatty acids metabolism. [37]
Borage Seed Oil
Borage (Borago officinalis), originally from the Mediterranean, has been used from ancient times for culinary and medicinal purposes.[38] They are believed to be the richest known source of Gamma-Linolenic acid (GLA), an essential omega-6 polyunsaturated fatty acid.[39]
The oil from borage seeds has shown benefits for treating different diseases, including atopic dermatitis, diabetic neuropathy, premenstrual syndrome, rheumatoid arthritis, and menopause-related symptoms.[40],[41]
GLA plays an important role in modulating inflammation throughout the body, especially when incorporated into the membranes of immune system cells.[42]
Black currant seeds and evening primrose oils also contain GLA. I recommend a fatty acid blend that includes high-quality EPA (eicosapentaenoic acid)/DHA (docosahexaenoic acid) omega-3 fish oil, together with EPA-rich borage oil and Sea Buckthorn oil.
Stick With Borage Oil, Not Pure GLA
GLA-rich borage oil is an underutilized supplement in cancer patient support protocols. Borage oil exerts selective cytotoxic effects without affecting normal healthy cell metabolism.
We hear a lot about how omega-6-polyunsaturated fatty acid (omega-6 PUFA)-rich diet has a pro-tumor effect, whereas an omega-3-rich diet has an anti-tumor effect.[43]
But I think this is misleading. The issue here is that the majority of omega-6 fatty acids come from processed and often rancid foods, fried foods using GMO corn oil.
Ginkgo Biloba
Extract from Ginkgo biloba has been demonstrated to inhibit fatty acid synthase activity. It also appears to be toxic to human cancer cells.
Cancer is capable of manipulation. Unlike healthy cell metabolism, cancer cells take control of any and all components of energy metabolism. In order to stop cancer, we have to stop unchecked growth.
Ginkgo biloba extract inhibits FAS activity and demonstrates cytotoxic activity in human cancer cells.[44] Ginkgolic acid, an active compound within Ginkgo biloba, specifically inhibits the expression of FAS, which suggested alkylphenol derivatives a new type of FAS inhibitor.[45]
Inhibits the inducible PGE2 synthases and cancer-related inflammation
Ginkgolic acid is a multi-target inhibitor of key enzymes in pro-inflammatory lipid mediator biosynthesis.[46] Ginkgolides A, B, and C also contribute to the anti-tumor effect of ginkgo through different mechanisms. Ginkgolide A reduced neointimal hyperplasia, a hyperproliferative state of vascular smooth muscle cells by decreasing Erk1/2 signal transduction. Ginkgolide B induced autophagy in lung cancer cells and inhibited NLRP3 inflammasome. This is relevant in the context of cancer because inflammation promoted by the NLRP3 inflammasome can lead to cancer and promote the growth of cancer.[iv]Ginkgolic acid, also exhibit antiviral activity[47] and was recently found to inhibit SARS-CoV-2 cysteine proteases.[48]
Botanical and Dietary Medicine to Reestablish Healthy Cell Metabolism
Boosting our healthy cell metabolism with botanical and dietary medicine can sort this out. Using botanical medicine helps steer the network of metabolic processes in favor of healthy cell metabolism and away from cancer cell metabolism.
These plants act as metabolic re-programmers. Eat them! Improve your diet with high-quality supplements! Work with a knowledgeable healer who can help you use the arsenal of natural medicine to reestablish healthy cell metabolism.
About the Author:
Donald R. Yance is the founder of the Mederi Center. A Clinical Master Herbalist and Certified Nutritionist, Donnie is renowned for his extraordinary knowledge and deep understanding of the healing properties of plants and nutrition, as well as of epigenetics, laboratory medicine, oncologic pathology, and molecular oncology. He is a professional member of the American Herbalists Guild, National Association of Nutrition Professionals, Academy of Integrative Health and Medicine, and the Society for Integrative Oncology.
[1] Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK. Autophagy and apoptosis: where do they meet? Apoptosis. 2014 Apr;19(4):555-66. doi: 10.1007/s10495-014-0967-2. PMID: 24415198.
[2] Gupta R, Ambasta RK, Pravir Kumar. Autophagy and apoptosis cascade: which is more prominent in neuronal death? Cell Mol Life Sci. 2021 Dec;78(24):8001-8047. doi: 10.1007/s00018-021-04004-4. Epub 2021 Nov 6. PMID: 34741624.
[3] H. Lee Moffitt Cancer Center & Research Institute. “How cancer cells ‘hijack’ a mechanism to grow.” ScienceDaily. ScienceDaily, 14 August 2012. <www.sciencedaily.com/releases/2012/08/120814100254.htm>.
[4] Borden KLB. Cancer cells hijack RNA processing to rewrite the message. Biochem Soc Trans. 2022 Oct 31;50(5):1447-1456. doi: 10.1042/BST20220621. PMID: 36282006; PMCID: PMC9704515.
[5] Srivastava A, Srivastava P, Mathur S, Abbas S, Rai N, Tiwari S, Tiwari M, Sharma L. Lipid Metabolism and Mitochondria: Cross Talk in Cancer. Curr Drug Targets. 2022;23(6):606-627. doi: 10.2174/1389450122666210824144907. PMID: 34431462.
[6] Fnu G, Weber GF. Alterations of Ion Homeostasis in Cancer Metastasis: Implications for Treatment. Front Oncol. 2021 Dec 20;11:765329. doi: 10.3389/fonc.2021.765329. PMID: 34988012; PMCID: PMC8721045.
[7] Jin Z, Chai YD, Hu S. Fatty Acid Metabolism and Cancer. Adv Exp Med Biol. 2021;1280:231-241. doi: 10.1007/978-3-030-51652-9_16. PMID: 33791986.
[8] Srivastava A, Srivastava P, Mathur S, Abbas S, Rai N, Tiwari S, Tiwari M, Sharma L. Lipid Metabolism and Mitochondria: Cross Talk in Cancer. Curr Drug Targets. 2022;23(6):606-627. doi: 10.2174/1389450122666210824144907. PMID: 34431462https://pubmed.ncbi.nlm.nih.gov/34431462/.
[9] Jin Z, Chai YD, Hu S. Fatty Acid Metabolism and Cancer. Adv Exp Med Biol. 2021;1280:231-241. doi: 10.1007/978-3-030-51652-9_16. PMID: 33791986.
[10] Ma Y, Temkin SM, Hawkridge AM, Guo C, Wang W, Wang XY, Fang X. Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer Lett. 2018 Oct 28;435:92-100. doi: 10.1016/j.canlet.2018.08.006. Epub 2018 Aug 10. PMID: 30102953; PMCID: PMC6240910.
[11] Zhang C, Zhu N, Li H, Gong Y, Gu J, Shi Y, Liao D, Wang W, Dai A, Qin L. New dawn for cancer cell death: Emerging role of lipid metabolism. Mol Metab. 2022 Sep;63:101529. doi: 10.1016/j.molmet.2022.101529. Epub 2022 Jun 15. PMID: 35714911; PMCID: PMC9237930.
[12] Butler LM, Perone Y, Dehairs J, Lupien LE, de Laat V, Talebi A, Loda M, Kinlaw WB, Swinnen JV. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv Drug Deliv Rev. 2020;159:245-293. doi: 10.1016/j.addr.2020.07.013.
[13] Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, Pinnick KE, Wigfield S, Buffa FM, Li JL, Zhang Q, Wakelam MJO, Karpe F, Schulze A, Harris AL, Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia- reoxygenation, Cell Rep, 9 (2014) 349–365.
[14] Bailey AP, Koster G, Guillermier C, Hirst EM, MacRae JI, Lechene CP, Postle AD, Gould AP, Antioxidant Role for Lipid Droplets in a Stem Cell Niche of Drosophila, Cell, 163 (2015) 340– 353
[15] Butler LM, Perone Y, Dehairs J, Lupien LE, de Laat V, Talebi A, Loda M, Kinlaw WB, Swinnen JV. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv Drug Deliv Rev. 2020;159:245-293. doi: 10.1016/j.addr.2020.07.013.
[16] Menendez JA, Decker JP, Lupu R. In support of fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem. 2005 Jan 1;94(1):1-4. doi: 10.1002/jcb.20310. PMID: 15523670.
[17] Ioachim HL, Decuseara R, Giancotti F, Dorsett BH. FAS and FAS-L expression by tumor cells and lymphocytes in breast carcinomas and their lymph node metastases. Pathol Res Pract. 2005;200(11-12):743-51. doi: 10.1016/j.prp.2004.09.006.
[18] Tang Y, Zhou J, Hooi SC, Jiang YM, Lu GD. Fatty acid activation in carcinogenesis and cancer development: Essential roles of long-chain acyl-CoA synthetases. Oncol Lett. 2018 Aug;16(2):1390-1396. doi: 10.3892/ol.2018.8843. Epub 2018 May 30. PMID: 30008815; PMCID: PMC6036470.
[19] Carvalho MA, Zecchin KG, Seguin F, Bastos DC, Agostini M, Rangel AL, Veiga SS, Raposo HF, Oliveira HCF, Loda M, et al. (2008). Fatty acid synthase inhibition with orlistat promotes apoptosis and reduces cell growth and lymph node metastasis in a mouse melanoma model. Int J Cancer. 123:2557–65.
[20] Pflug BR, Pecher SM, Brink AW, Nelson JB, Foster BA. Increased fatty acid synthase expression and activity during progression of prostate cancer in the TRAMP model. Prostate. 2003 Nov 1;57(3):245-54. doi: 10.1002/pros.10297. PMID: 14518031.
[21] Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004 Jan 1;91(1):47-53. doi: 10.1002/jcb.10708. PMID: 14689581.
[22] Murata S, Yanagisawa K, Fukunaga K, Oda T, Kobayashi A, Sasaki R, Ohkohchi N. (2010). Fatty acid synthase inhibitor cerulenin suppresses liver metastasis of colon cancer in mice. Cancer Sci. 101:1861–5.
[23] Zhang C, Zhu N, Li H, Gong Y, Gu J, Shi Y, Liao D, Wang W, Dai A, Qin L. New dawn for cancer cell death: Emerging role of lipid metabolism. Mol Metab. 2022 Sep;63:101529. doi: 10.1016/j.molmet.2022.101529. Epub 2022 Jun 15. PMID: 35714911; PMCID: PMC9237930.
[24] Zhang C, Zhu N, Li H, Gong Y, Gu J, Shi Y, Liao D, Wang W, Dai A, Qin L. New dawn for cancer cell death: Emerging role of lipid metabolism. Mol Metab. 2022 Sep;63:101529. doi: 10.1016/j.molmet.2022.101529. Epub 2022 Jun 15. PMID: 35714911; PMCID: PMC9237930.
[25] Cheng C, Zhuo S, Zhang B, Zhao X, Liu Y, Liao C, Quan J, Li Z, Bode AM, Cao Y, Luo X. Treatment implications of natural compounds targeting lipid metabolism in nonalcoholic fatty liver disease, obesity and cancer. Int J Biol Sci. 2019 Jun 4;15(8):1654-1663. doi: 10.7150/ijbs.33837. PMID: 31360108; PMCID: PMC6643217.
[26] Y Lin, M A Vermeer, W Bos. et al. Molecular structures of citrus flavonoids determine their effects on lipid metabolism in HepG2 cells by primarily suppressing apoB secretion. J Agric Food Chem. 2011;59(9):4496–503
[27] N M Borradaile, L E de Dreu, M W Huff. Inhibition of net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes. 2003;52(10):2554–61.
[28] Pandey PR, Okuda H, Watabe M, Pai SK, Liu W, Kobayashi A, Xing F, Fukuda K, Hirota S, Sugai T, Wakabayashi G, Koeda K, Kashiwaba M, Suzuki K, Chiba T, Endo M, Fujioka T, Tanji S, Mo YY, Cao D, Wilber AC, Watabe K. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Cancer Res Treat. 2011 Nov;130(2):387-98. doi: 10.1007/s10549-010-1300-6. Epub 2010 Dec 29. PMID: 21188630; PMCID: PMC3404809.
[29] Zeng YW, Yang JZ, Pu XY, Du J, Yang T, Yang SM, Zhu WH. (2013). Strategies of functional food for cancer prevention in human beings. Asian Pac J Cancer Prev. 14:585–592.
[30] Tian WX. (2006). Inhibition of fatty acid synthase by polyphenols. Curr Med Chem. 13:967–77.
[31] Lakhanpal P, Rai DK. Quercetin: a versatile flavonoid. Internet J Med Update. 2007;2(2):22–37
[32] Vafadar A, Shabaninejad Z, Movahedpour A, Fallahi F, Taghavipour M, Ghasemi Y, Akbari M, Shafiee A, Hajighadimi S, Moradizarmehri S, Razi E, Savardashtaki A, Mirzaei H. Quercetin and cancer: new insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020 Mar 10;10:32. doi: 10.1186/s13578-020-00397-0. PMID: 32175075; PMCID: PMC7063794.
[33] Vargas AJ, Burd R. Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr Rev. 2010;68(7):418–428.
[34] D Porras, E Nistal, S Martinez-Florez. et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic Biol Med. 2017;102:188–202.
[35] A K Stoldt, M Mielenz, G Nurnberg. et al. Effects of a six-week intraduodenal supplementation with quercetin on liver lipid metabolism and oxidative stress in peripartal dairy cows. J Anim Sci. 2016;94(5):1913–23
[36] Sur S, Nakanishi H, Flaveny C, Ippolito JE, McHowat J, Ford DA, Ray RB. Inhibition of the key metabolic pathways, glycolysis and lipogenesis, of oral cancer by bitter melon extract. Cell Commun Signal. 2019 Oct 21;17(1):131. doi: 10.1186/s12964-019-0447-y. Erratum in: Cell Commun Signal. 2019 Nov 19;17(1):151.
[37] Wang S, Hao HF, Jiao YN, Fu JL, Guo ZW, Guo Y, Yuan Y, Li PP, Han SY. Dandelion extract inhibits triple-negative breast cancer cell proliferation by interfering with glycerophospholipids and unsaturated fatty acids metabolism. Front Pharmacol. 2022 Sep 6;13:942996. doi: 10.3389/fphar.2022.942996. PMID: 36147318; PMCID: PMC9486077.
[38] Gerard J. In: The History of Plants, 1597. Woodward M., editor. Senate. Study Ltd.; London, UK: 1994. pp. 185–186.
[39] Janick J., Simon J.E., Quinn J., Beaubaire N. Borage: A Source of Gamma Linolenic Acid. Herbs Spices Med. Plants Recent Adv. Bot. Hortic. Pharmacol. 1989;4:145–168.
[40] Cameron M., Gagnier J.J., Chrubasik S. Herbal Therapy for Treating Rheumatoid Arthritis. Cochrane Database Syst. Rev. 2011;16:CD002948. doi: 10.1002/14651858.CD002948.pub2.
[41] an Y.-Y., Chapkin R.S. Importance of Dietary γ-Linolenic Acid in Human Health and Nutrition. J. Nutr. 1998;128:1411–1414. doi: 10.1093/jn/128.9.1411.
[42] Johnson MM, Swan DD, Surette ME, et al. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J Nutr. 1997 Aug;127(8):1435-44.
[43] Montecillo-Aguado M, Tirado-Rodriguez B, Tong Z, Vega OM, Morales-Martínez M, Abkenari S, Pedraza-Chaverri J, Huerta-Yepez S. Importance of the Role of ω-3 and ω-6 Polyunsaturated Fatty Acids in the Progression of Brain Cancer. Brain Sci. 2020 Jun 17;10(6):381. doi: 10.3390/brainsci10060381. PMID: 32560280; PMCID: PMC7349634.
[44] Oh J, Hwang IH, Hong CE, Lyu SY, Na M. Inhibition of fatty acid synthase by ginkgolic acids from the leaves of Ginkgo biloba and their cytotoxic activity. J Enzyme Inhib Med Chem. 2013 Jun;28(3):565-8. doi: 10.3109/14756366.2012.658786. Epub 2012 Mar 1. PMID: 22380770.
[45] Oh J, Hwang IH, Hong CE, Lyu SY, Na M. (2013). Inhibition of fatty acid synthase by ginkgolic acids from the leaves of Ginkgo biloba and their cytotoxic activity. J Enzyme Inhib Med Chem. 28:565–8.
[46] Gerstmeier J, Seegers J, Witt F, Waltenberger B, Temml V, Rollinger JM, Stuppner H, Koeberle A, Schuster D, Werz O. Ginkgolic Acid is a Multi-Target Inhibitor of Key Enzymes in Pro-Inflammatory Lipid Mediator Biosynthesis. Front Pharmacol. 2019 Jul 17;10:797. doi: 10.3389/fphar.2019.00797. PMID: 31379572; PMCID: PMC6650749.
[47] Wang, X.; Shao, Q.H.; Zhou, H.; Wu, J.L.; Quan, W.Q.; Ji, P.; Yao, Y.W.; Li, D.; Sun, Z.J. Ginkgolide B inhibits lung cancer cells promotion via beclin-1-dependent autophagy. BMC Complement. Med. 2020, 20, 194
[48] Bhutta MS, Shechter O, Gallo ES, Martin SD, Jones E, Doncel GF, Borenstein R. Ginkgolic Acid Inhibits Herpes Simplex Virus Type 1 Skin Infection and Prevents Zosteriform Spread in Mice. Viruses. 2021 Jan 9;13(1):86. doi: 10.3390/v13010086. PMID: 33435520; PMCID: PMC7826900.
[49] Chen, Z., Cui, Q., Cooper, L. et al. Ginkgolic acid and anacardic acid are specific covalent inhibitors of SARS-CoV-2 cysteine proteases. Cell Biosci 11, 45 (2021). https://doi.org/10.1186/s13578-021-00564-x