In the aggressive response to the global health emergency, many are having a hard time trying to find reliable data to effectively address a host of concerns about the virus itself, as well as the short and long-term risks associated with the new vaccines (currently Moderna and Pfizer), especially due to their origins (mRNA), potential adverse effects, and whether or not they will be effective against the new variants. There are many questions and concerns, and not a lot of data, given the short time the pandemic and the vaccines have been in existence. Many are also wondering whether or not they are protected from the virus if they have already gotten it and have built antibodies. The media has led us to believe that natural immunity is not effective at preventing a second infection however, according to the scientific data, natural immunity is highly protective, at least in the first six months to a year (the time frame for which we have data).[1],[2] COVID-19 continues to be an evolving disease that requires a sophisticated and myriad approach. A wholistic framework employing traditional herbal medicines and key nutrients to support the immune system and mitigate side effects is an important and under-recognized strategy in this complex environment. Several Asian countries are studying herbal medicines in COVID-19 as part of a multifaceted approach to control the severity and morbidity of the disease.
Vaccine Concerns
While the vaccines obviously provide the clear benefit of immunity to the virus, there are some potential side effects that should be acknowledged. First of all, the anaphylaxis rate with COVID-19 vaccinations is 10 times greater than for flu shots. Allergic reactions to both the Moderna and Pfizer COVID-19 have been reported with severe allergies in the U.S. and U.K. Most allergies would not cause extreme reactions to the COVID-19 vaccine, according to the Centers for Disease Control.[3] Anyone with a history of immediate allergic reaction of any severity to any component of mRNA COVID-19 vaccines or to polysorbate should not be vaccinated, the CDC said.[4] Polysorbate is not a vaccine ingredient but was included because people sensitive to it may also react to polyethylene glycol.
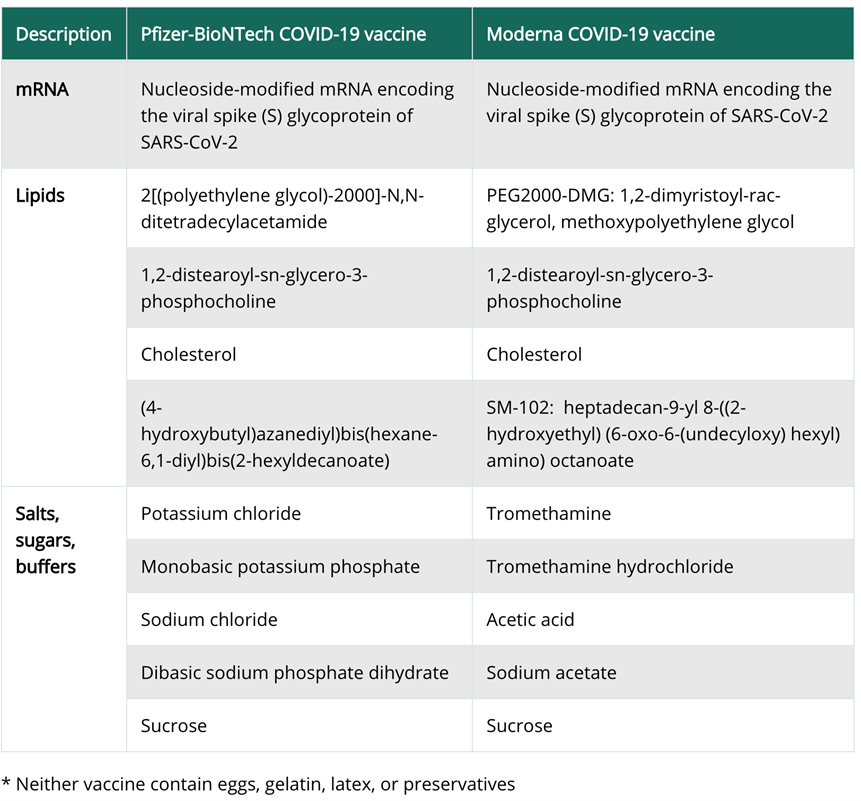
Solicited adverse events that occurred in more than half the participants included fatigue, chills, headache, myalgia, and pain at the injection site. Systemic adverse events were more common after the second vaccination.[6]
Additionally, COVID-19 vaccinations have been associated with adverse drug reactions in elderly people who are frail. As of 14 January, 23 reports of suspected deaths associated with COVID-19 vaccines have been submitted to the Norwegian health registry. At this time we don’t know if the vaccination itself had any contribution to any of these deaths, but these vaccines have not really been tested in the most variable group – the elderly. “The reports suggest that common adverse reactions to mRNA vaccines, such as fever and nausea, may have contributed to a fatal outcome in some frail patients”, says Sigurd Hortemo, chief physician at the Norwegian Medicines Agency.[7]
Furthermore, there are other potential long-term side effects of the vaccine that have yet to be illuminated. As these vaccines utilize a brand-new technology and brand-new ingredients, it would be inaccurate to say that we are entirely sure of their long-term ramifications to our health.
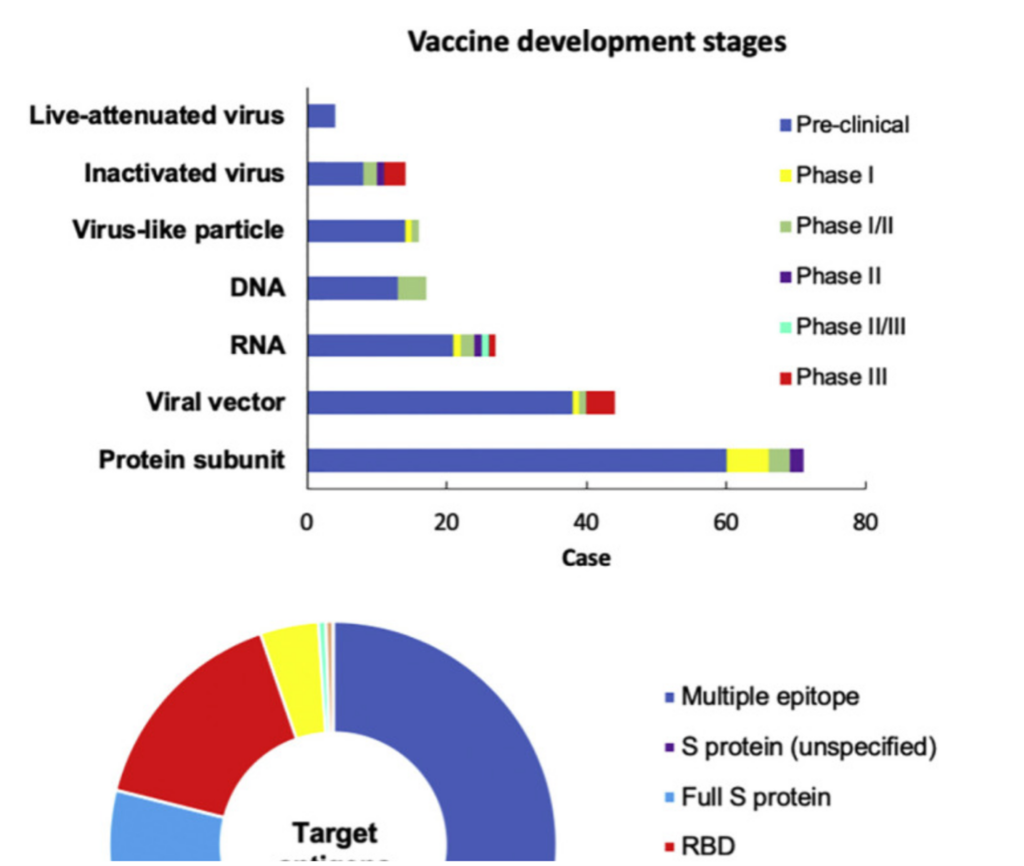
Current pre-and clinical trials of COVID-19 vaccines, according to World Health Organization as of December 8, 2020. Out of 214 vaccine candidates, 52 are in clinical trials and 162 are in pre-clinical studies.
Most of these platforms have not been licensed for use in humans yet, leading to questions of long-term safety as well as the degree to which they can induce strong and long-term immunity.[8] An additional key concern is relying on the “S-only” vaccines, as mutations have been detected in the spike (S) protein of SARS-CoV-2.[9],[10]
Some of these viral variants may not depend on the angiotensin-converting enzyme 2 (ACE2) for entry, which would mean the vaccines will not work. In the widely circulating virus, the densely glycosylated spike (S) protein on its surface engages ACE2 to mediate host cell entry. However, new studies have reported viral susceptibility in intra- and extra pulmonary immune and non-immune cells lacking ACE2, suggesting that the S protein may exploit additional receptors for infection. Some of these include interactions between S protein and the immune C-lectin type receptors, toll-like receptors and neuropilin-1, and the non-immune receptor glucose regulated protein 78.[11]
The New South African SARS-CoV-2 Variant
This new South African (501Y.V2) variant is characterized by three mutations in the SARS-CoV-2 spike (S) protein: K417N, E484K and N501Y. This latest mutation is also present in the UK (VUI-202,012/01) variant.
Although, this variant is considered to be a more highly transmissible strain due to the rapidity with which it became predominant in this South African population over just a few weeks, there appears to be no indication of increased severity of illness, as yet, though research is ongoing in both the UK and South Africa to further investigate this phenotype variant.[12]
Johnson & Johnson’s COVID-19 One-dose Vaccine Soon to be Approved
Johnson & Johnson’s COVID-19 one-dose vaccine candidate was 66% effective overall against moderate to severe COVID-19 in a multinational phase III trial, and 72% effective in the U.S. cohort.
In South Africa, where the Johnson & Johnson’s single-dose vaccine was tested,nearly all cases were due to infection with a variant from the B.1.351 lineage. Although the company said the vaccine was generally protective there, too, the lower rates of protection most likely reflect this different variant.[13] New data shows, that the Johnson & Johnson’s COVID-19 vaccine appears to be extremely (85%) effective against severe disease.[14]
The product, developed by Johnson & Johnson’s Janssen unit, differs from other approved vaccines in that it uses an adenoviral vector to deliver genetic material encoding coronavirus spike protein elements. This AdVac® technology platform is stable for months at refrigerator temperatures.
The AdVac® technology platform was also used to develop and manufacture Janssen’s European Commission approved Ebola vaccine and construct its Zika, RSV, and HIV vaccine candidates. Janssen’s AdVac® technology platform has been used to vaccinate more than 100,000 people to date across Janssen’s investigational vaccine programs.[15]
Understanding Immune Response to SARS-CoV-2 at Varying Pathological Stages
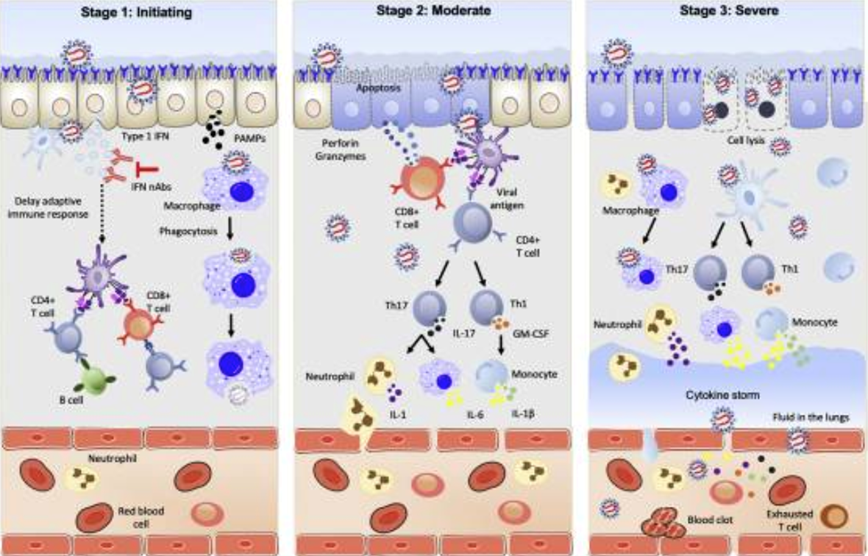
In stage 1, in the nasal cavity, SARS-CoV-2 binds to ACE2 receptors on epithelial cells by the S protein, enters the cell, and starts to replicate. The virus proliferates and simultaneously travels down the respiratory tract, and clinical manifestation of symptoms start appearing. Upon infection, the virus activates type I interferons. In COVID-19 patients, type 1 interferon production is delayed by SARS-CoV-2, which lowers the adaptive immune response. The host innate immune system detects SARS-CoV-2 via recognition of pathogen-associated molecular patterns (PAMPs) by the alveolar macrophages activating cytokines such IL-6 and TNF-α leading to phagocytosis of the virus or activation.
In stage 2, infected epithelial cells present viral antigens to both CD4+ and CD8+ T cells. CD8+ T cells release perforins and granzymes that induce apoptosis of the infected cells. CD4+ T cells rapidly activate to become Th1 cells that secrete granulocyte-macrophage colony-stimulating factor and further induce monocytes by high IL-6 levels. An increase in monocyte subpopulation promotes IL-1β production. Th17 cells produce IL-17 to further recruit monocytes, macrophages, and neutrophils, and stimulates other inflammatory cytokines such as IL-6, IL-1, and IL-1β.
In stage 3, inflammatory cells release additional cytokines which amplifies the cytokine storm and exacerbates the systemic inflammatory response, eventually leading to acute respiratory distress syndrome, multiorgan failure, and death.[16]
TCM Treatment for COVID-19
The Chinese government has claimed that combining herbal medicine with conventional medicine has helped the country deal with the outbreak. Last month, China’s National Health Commission issued a document regarding treating COVID-19 patients with several herbal medicines to relieve symptoms such as weakness and fever.
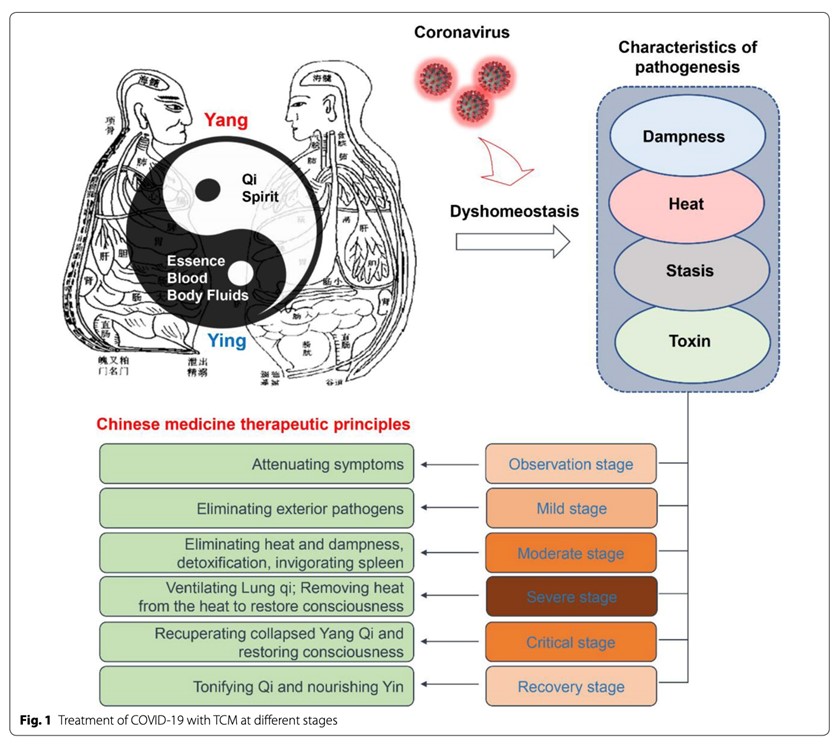
There are a variety of Traditional Chinese Medicine (TCM) prescriptions recommended for the treatment of COVID-19, including three prescriptions endorsed by the Chinese authorities during the pandemic.[17] The objective of acute symptom stage treatment from the perspective of TCM involves dispelling epidemic toxins. After acute stage treatment, patients move on to the recovery stage. The objective of the recovery phase is to restore vitality and strengthen the lungs, as well as strengthen any other organ systems that may have been affected, including the cardiovascular, immune and digestive systems.

A new study found that TCM treatment on the convalescents of COVID-19 was very useful. A total of 372 COVID-19 convalescents from February 21 to May 3, 2020 in Shenzhen, China were retrospectively analyzed, 291 of them accepted clinical examination at least once and 191 convalescents accepted TCM. The beneficial effects included balanced immune response, improved hematopoiesis and coagulation systems, enhanced functions of liver and heart, increased nutrient intake and lipid metabolism.[19]
In TCM, herbal medicine with heat-clearing and detoxifying or tonic effects are commonly used in the treatment of the lung and intestinal diseases by regulating lung-bowel mucosal immunity and they can be used to treat lung/intestinal conditions simultaneously.[20] The “lung and large intestine being interior-exteriorly related” is one of the classical theories in TCM, which indicates a close correlation between the lung and large intestine in physiology and pathology, and plays a pivotal role in guiding the treatment of the lung and bowel diseases.
J. Qi writes “The natural environment in different regions varies, which diversifies the pathogenesis of diseases and the treatment approaches. For example, Guangzhou province in southern China has a humid and hot climate with a higher degree of dampness. Therefore, Chinese medicines with eliminating dampness effects should be frequently used. However, in Gansu province with dry climate, it is suggested that patients also presented the characteristic of dampness pathogen similar to that in Wuhan.”[21]
Artemisia in Africa
Antimalarial artemisinin-based combination therapies are being used and studied for COVID-19 in Africa[22] Artemisia annua extracts prevent in vitro replication of SARS-CoV-2[23]]
When deaths rose as South Africa battled its first Covid-19 wave, people turned to traditional medicines, including Artemisia. Artemisia afra has been in high demand on the streets of Johannesburg. To treat Covid-19 symptoms, the indigenous herb’s silvery leaves were for sale at roadside vendors and in the city’s popular traditional markets. Some people even pulled the plant from private gardens. People in South Africa have consumed the bitter plant for centuries to treat illnesses from colds to intestinal worms and malaria.

In Africa, many herbal remedies are closely guarded secrets, intertwined with a philosophy in which health is inextricably linked with spiritual life. And unlike other ancient health care systems that rely on written texts, African healers share and preserve knowledge largely through oral tradition, so there is little record of how the medicines were made and used hundreds of years ago.
Artemisia is one of several herbs that the African government is investigating against Covid-19. In July of 2020, officials set up the African Medicines Covid-19 Research Team, which includes scientists and traditional healers, and diverted about $880,500 from existing indigenous knowledge projects to fund the collaboration.[24]
Also, Dr. Monica Musenero, the Presidential Advisor on Epidemics, who is leading the team of researchers in Africa, recently stated that they are finalizing documentation of a study of an herbal medicine blend to treat Covid-19, which will be submitted to Uganda National Council for Science and Technology. Soon they will begin treating patients with the medicine.[25]
Andrographis in Thailand
The government of Thailand has approved the use of the traditional herb, andrographis (Andrographis paniculata) in a pilot study aimed at reducing the initial symptoms and subsequent severity of COVID-infected individuals. Andrographis has been clinically used for inflammatory diseases and anti-viral therapy for centuries.

Andrographis Paniculata
“Andrographis will serve as an alternative treatment to reduce the severity of the outbreak and cut treatment costs” the ministry said in a statement. Human trials have shown patient conditions improved within three days of the treatment without side effects if the medicine is administered within 72 hours of testing positive. The treatment will be available in five state-owned hospital initially.”[26]
Andrographolide is a diterpenoid isolated from Andrographis, which has been employed in treating various diseases such as cancer and inflammatory diseases.[27] Andrographolide is responsible for a wide range of biological activities such as antioxidant, anti-inflammatory, and anti-cancer properties.[28] Andrographolide, as well as cyclocurcumin and curcumin from Turmeric, significantly binds with the active site of SARS CoV-2 main protease.[29]
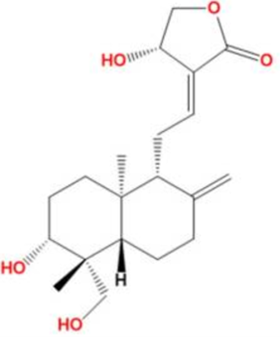
Andrographolide
Andrographolides have been found to reduce inflammation significantly in several animal models.[30] Andrographolides, are able to down-modulate both humoral and cellular adaptive immune responses in over-active pro-inflammatory conditions. Andrographolide exhibits immunomodulatory effect by increasing the level of cytotoxic T cells, NK cells, phagocytic cells, and shows the broad spectral antiviral activity against various deadly viruses.[31] Andrographolide has also been shown to interfere with T cell proliferation and cytokine release in response to allergenic stimulation in an autoimmune in both laboratory and animal models.[32],[33] Andrographolides possess anti-viral activity as well, showing to inhibit the expression of Epstein-Barr virus.[34]
Recent computational analysis data suggest that Andrographolide produces therapeutic effects against severe acute respiratory syndrome coronavirus (SARS-CoV) pneumonia infections.[35] Andrographolide suppresses the main protease activities of 2019-nCoV and SARS-CoV. Considering that andrographolide is used in clinical practice with acceptable safety and its diverse pharmacological activities could be beneficial for attenuating COVID-19 symptoms, extensive investigation of andrographolide on the suppression of 2019-nCoV as well as its application in COVID-19 therapy has been suggested.[36] The toxicity prediction of the compounds in Andrographis also reveals their safety. These results confirm the probability of using Andrographis phytochemical compounds as COVID-19 main protease inhibitors.[37]
The aerial parts and roots of andrographis have been widely used as traditional medicine in China, India, Thailand and other Southeast Asian countries to treat many maladies. It is known as King of Bitters (English), Mahatikta (Sanskrit), Kiryato (Gujarati), Mahatita (Hindi), Kalmegh (Bengali), or Fah Talai Jone (Thai).[38] In traditional Chinese medicine (TCM) it is referred to as Chuanxinlian, Yijianxi or Lanhelian which has been long known as an immune system modulator to remove toxic heat (inflammation). Andrographis has demonstrated significant activity in fighting the common cold, flu and upper respiratory infections.[39]
Andrographis and Its Active Chemical Compounds Andrographolides have Shown:
- Liver protection and reduced liver inflammation.[40],[41],[42],[43]
- Cardio-protection: protects cardiomyocytes against hypoxia/reoxygenation injury[44]
- Significant inhibition of thrombin-induced platelet aggregation, [45] and platelet-activating factor [46],[47]
- Protection against atherosclerosis [48]
- Neuroprotection[49]
- Immune modulation – Increase T-cell activity by enhancement of IL-2 and INF-gamma[50],[51]
- Pain/inflammation inhibition[52]
- Redox/anti-oxidation – Directly neutralizes free radicals by protecting mitochondrial integrity, inhibiting pro-oxidant enzymes, and/or activating antioxidant enzymes[53]
- Anti-ulcer/GI protection[54]
TCM Energetic Attributes of Andrographis:
Nature: Bitter, cold
Enters: Small Intestine, Large Intestine, Lung, Stomach
Actions: Dries dampness, clears heat, purges fire and removes toxicity[55]
I do not recommend andrographis to be taken as a single remedy. Energetically, it is a cold and bitter herb and by itself may not be the best suited for intimal presenting symptoms. It is contraindicated in patients with deficiency cold syndrome unless it is combined with herbs to mitigate this effect.
In the initial stage of Covid-19, there are many other herbs I would think of before andrographis. However, it is a major herb I would suggest for people who are not getting well quickly and are moving into the 2nd stage, which includes prolonged inflammation of the lungs or bowels.
Due to complicated pathogenesis, a single herb often fails to meet the clinical needs and get a desired effect. Therefore, herbs should be combined for comprehensive effects.[56] I like to combine andrographis with other herbs including Magnolia spp, Boswellia serrata, Chinese skullcap (Scutellaria baicalensis), and Feverfew (Tanacetum parthenium). I find these herbs in combination to be very helpful for heat conditions of the lungs, bowels, liver, and muscle skeletal system.
Nutrients to Optimize the Immune System
It is becoming apparent that vitamin D and zinc deficiency are significant morbidity and mortality risk factors in COVID-19. The response to the pandemic should include an effort to aggressively eliminate these deficiencies.
Vitamin D deficiency, associated with deleterious effects on innate and adaptive immunity, has many growing datasets that point to it as a risk factor for severe COVID-19. Hypovitaminosis D is very common, occurring in 75% of patients and there is significant association with in-hospital mortality in a dose-effect manner.[57] COVID-19 Patients with poor prognosis have significantly lower serum levels of vitamin D compared with those with good prognosis.[58]
Additionally, zinc deficiency places the individual at an increased risk of infections and secondary complications, delayed recovery, decreased wound healing, and increased vulnerability to cell damage from the acute phase response. There is a considerable overlap between the symptomatology of SARS CoV-2 infection and zinc deficiency. Some of the unusual COVID-19 symptoms such as loss of smell and taste are well established in conjunction with zinc deficiency. All adults could be supplemented with up to a maximum of 50 mg/day without significant toxic side-effects.[59]
Both zinc and vitamin D can easily be supplemented in cases of deficiency. Everyone should be checking serum 25-OH-D levels, 125 diOH D levels, zinc, and selenium levels, yet its mostly not even recommended.
Quercetin
Additionally, Quercetin is a common plant flavonoid that can regulate the Th1/Th2 stability and decrease the antigen-specific IgE antibody releasing by B cells. Quercetin has a main role in anti-inflammatory and immunomodulatory function, which makes it proper for the management of different diseases.
Quercetin’s anti-allergenic effects
Quercetin may be useful for those getting the vaccine, both as a general immune supportive agent and possibly as a way to reduce the potential for allergic reactions.[60] Quercetin could be at least partially effective at suppressing the allergic reaction because its more effective than cromolyn in blocking human mast cell cytokine release.[61]
According to epidemiological, experimental and observational studies, many case reports and several intervention studies show supplementation with vitamin D, zinc, quercetin and possibly selenium are promising. The multiple anti-inflammatory and immunomodulatory effects of supplementation with these nutrients (not as a treatment but to optimize innate immune health) could explain their protective role against immune hyper reaction and cytokine storm in patients with severe COVID-19. “A randomized, placebo-controlled intervention study even shows that high dose vitamin D supplementation promotes viral clearance in asymptomatic and mildly symptomatic SARS-CoV-2 positive individuals. Besides, the data of a recent prospective study with COVID-19 patients reveal that a significant number of them were zinc deficient. The zinc deficient patients had more complications and the deficiency was associated with a prolonged hospital stay and increased mortality. Thus, immune-relevant micronutrients may help to increase the physiological resilience against COVID-19.”[62]
SeleniumIn the southern part of India, the serum blood levels of selenium were analyzed in 30 apparently healthy individuals and in 30 patients with confirmed COVID-19 infection. The patients showed significantly lower selenium levels of 69.2 ± 8.7 ng/mL than controls 79.1 ± 10.9 ng/mL. The difference was statistically significant (P = 0.0003). Interestingly, the control group showed a borderline level of selenium, suggesting that the level of this micronutrient is not optimum in the population studied.[63]
Selenium has an ongoing history of reducing the incidence and severity of various viral infections; for example, a German study found selenium status to be significantly higher in serum samples from surviving than non-surviving COVID-19 patients. Furthermore, a significant, positive, linear association was found between the cure rate of Chinese patients with COVID-19 and regional selenium status.
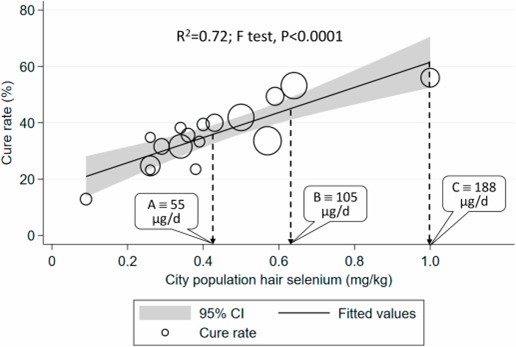
Correlation between COVID-19 cure rate in 17 cities outside Hubei on Feb 18, 2020, and city population selenium status (hair selenium concentration) analyzed using weighted linear regression. Each data point represents the cure rate, calculated as percentage of patients hospitalized with SARS-CoV-2 deemed to be cured. The size of the marker is proportional to the number of cases.
There is a significantly reduced expression of a number of selenoproteins, along with increased expression of IL-6 in SARS-CoV-2 infected cells in culture suggests a potential link between reduced selenoprotein expression and COVID-19-associated inflammation.
Proposed mechanism by which supra-nutritional levels of selenium might suppress the life cycle and mutation to virulence of SARS-COV-2 while attenuating viral-induced oxidative stress, organ damage and the cytokine storm.
There appears to be several tactics that SARS-CoV-2 could employ to evade an adequate host response by interfering with the human selenoprotein system.[64]
Final Thoughts
Herbal and nutritional medicines provide a global benefit to optimizing health and bolstering our immunity against viral pathogens and are an important part of an offensive strategy amid the fear and uncertainty of the pandemic now and into the future.
[1] Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, Marsden BD, Cox S, James T, Warren F, Peck LJ, Ritter TG, de Toledo Z, Warren L, Axten D, Cornall RJ, Jones EY, Stuart DI, Screaton G, Ebner D, Hoosdally S, Chand M, Crook DW, O’Donnell AM, Conlon CP, Pouwels KB, Walker AS, Peto TEA, Hopkins S, Walker TM, Jeffery K, Eyre DW; Oxford University Hospitals Staff Testing Group. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N Engl J Med. 2020 Dec 23:NEJMoa2034545. doi: 10.1056/NEJMoa2034545. Epub ahead of print. PMID: 33369366; PMCID: PMC7781098.
[2] Breathnach AS, Riley PA, Cotter MP, Houston AC, Habibi MS, Planche TD. Prior COVID-19 significantly reduces the risk of subsequent infection, but reinfections are seen after eight months. J Infect. 2021 Jan 13:S0163-4453(21)00010-4. doi: 10.1016/j.jinf.2021.01.005. Epub ahead of print. PMID: 33450303; PMCID: PMC7804382.
[3] https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html, retrieved 01/13/2020
[4] Medscape, Walker, Molly, 02/06/2021; https://www.medpagetoday.com/infectiousdisease/covid19/90556, retrieved 02/12/2021
[5] CDC COVID-19 Response Team, FDA “Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine — United States, December 14–23, 2020” MMWR 2021; Published Jan. 6, 2020.
[6] Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KS, Morabito KM, O’Dell S, Schmidt SD, Swanson PA 2nd, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH; mRNA-1273 Study Group. An mRNA Vaccine against SARS-CoV-2 – Preliminary Report. N Engl J Med. 2020 Nov 12;383(20):1920-1931.
[7] Covid-19 vaccination associated with adverse drug reactions in elderly people who are frail, https://legemiddelverket.no/nyheter/covid-19-vaccination-associated-with-deaths-in-elderly-people-who-are-frail, Statens legemiddelverk, 01/18/2020, retrieved 01/182020
[8] N. Biotechnology Whither COVID-19 vaccines? 2020. https://www.nature.com/articles/s41587-020-0697-7/ (Accessed 28 2020)
[9] Zhang L., Jackson C.B., Mou H., Ojha A., Rangarajan E.S., Izard T., Farzan M., Choe H. The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. Nat Commun. 2020;11:6013
[10] Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., Sheffield C.-G.G., McDanal C., Perez L.G., Tang H., Moon-Walker A., Whelan S.P., LaBranche C.C., Saphire E.O., Montefiori D.C. Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827
[11] Gadanec LK, McSweeney KR, Qaradakhi T, Ali B, Zulli A, Apostolopoulos V. Can SARS-CoV-2 Virus Use Multiple Receptors to Enter Host Cells? Int J Mol Sci. 2021 Jan 20;22(3):E992. doi: 10.3390/ijms22030992. PMID: 33498183.
[12] Tang JW, Toovey OTR, Harvey KN, Hui DDS. Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK [published online ahead of print, 2021 Jan 17]. J Infect. 2021;S0163-4453(21)00030-X. doi:10.1016/j.jinf.2021.01.007
[13] Ledford H. J&J’s one-shot COVID vaccine offers hope for faster protection. Nature. 2021 Jan 29. doi: 10.1038/d41586-021-00119-7. Epub ahead of print. PMID: 33526898.
[14] Janssen Vaccines & Prevention B.V.A Randomized, Double-blind, Placebo-controlled Phase 3 Study to Assess the Efficacy and Safety of Ad26.COV2.S for the Prevention of SARS-CoV-2-mediated COVID-19 in Adults Aged 18 Years and Older, Protocol VAC31518COV3001; Phase 3,AMENDMENT 3 VAC31518 (JNJ-78436735),December 20,2020
[15] Johnson & Johnson Announces Single-Shot Janssen COVID-19 Vaccine Candidate Met Primary Endpoints in Interim Analysis of its Phase 3 ENSEMBLE Trial, https://www.jnj.com/johnson-johnson-initiates-pivotal-global-phase-3-clinical-trial-of-janssens-covid-19-vaccine-candidate, 02/29/2020, study protocol https://www.jnj.com/coronavirus/covid-19-phase-3-study-clinical-protocol
[16] Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: The status and perspectives in delivery points of view [published online ahead of print, 2020 Dec 24]. Adv Drug Deliv Rev. 2020;170:1-25. doi:10.1016/j.addr.2020.12.011
[17] Yang Y. Use of herbal drugs to treat COVID-19 should be with caution. Lancet. 2020 May 30;395(10238):1689-1690. doi: 10.1016/S0140-6736(20)31143-0. Epub 2020 May 15. PMID: 32422123; PMCID: PMC7228688.
[18] Luo, H., Gao, Y., Zou, J. et al. Reflections on treatment of COVID-19 with traditional Chinese medicine. Chin Med 15, 94 (2020). https://doi.org/10.1186/s13020-020-00375-1
[19] An YW, Yuan B, Wang JC, Wang C, Liu TT, Song S, Liu HQ. Clinical characteristics and impacts of traditional Chinese medicine treatment on the convalescents of COVID-19. Int J Med Sci. 2021 Jan 1;18(3):646-651. doi: 10.7150/ijms.52664. PMID: 33437199; PMCID: PMC7797555.
[20] Lou Z, Zhao H, Lyu G. [Mechanism and intervention of mucosal immune regulation based on “lung and large intestine being interior-exteriorly related” theory of traditional Chinese medicine]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020 Dec 25;49(6):665-678. Chinese. doi: 10.3785/j.issn.1008-9292.2020.12.01. PMID: 33448169.
[21] Qi J, Qi X, Wang X. Clinical efficacy of different doses of jinhuaqinggan granule on influenza and serum levels of cytokines. Mod Med J. 2016;44:1664–9.
[22] Gendrot M, Duflot I, Boxberger M, Delandre O, Jardot P, Le Bideau M, Andreani J, Fonta I, Mosnier J, Rolland C, Hutter S, La Scola B, Pradines B. Antimalarial artemisinin-based combination therapies (ACT) and COVID-19 in Africa: In vitro inhibition of SARS-CoV-2 replication by mefloquine-artesunate. Int J Infect Dis. 2020 Oct;99:437-440. doi: 10.1016/j.ijid.2020.08.032. Epub 2020 Aug 14. PMID: 32805422; PMCID: PMC7426697.
[23] Nair MS, Huang Y, Fidock DA, Polyak SJ, Wagoner J, Towler MJ, Weathers PJ. Artemisia annua L. extracts prevent in vitro replication of SARS-CoV-2. bioRxiv [Preprint]. 2021 Jan 8:2021.01.08.425825. doi: 10.1101/2021.01.08.425825. PMID: 33442683; PMCID: PMC7805440.
[24] Wild, Sara, Bringing Traditional Healing Under the Microscope in South Africa – Medscape – Dec 31, 2020., https://www.medscape.com/viewarticle/943429_print, retrieved 01/20/2021
[25] Musenero, Dr. Monica, Study to examine effectiveness of Uganda’s herbal COVID-19 cure to start next week, 02/21/2021, https://www.independent.co.ug/study-to-examine-effectiveness-of-ugandas-herbal-covid-19-cure-to-start-next-week/, retrieved 02/21/2020
[26] Yuvejwattana, Suttinee,Thailand Clears Use of Herbal Medicine for Covid-19 Treatment, December 29, 2020, 10:02 PM PST, Bloomberg, https://www.bloomberg.com/news/articles/2020-12-30/thailand-clears-use-of-herbal-medicine-for-covid-19-treatment, retrieved 01/04/2021
[27] S. Rajagopal, R.A. Kumar, D.S. Deevi, C. Satyanarayana, R. RajagopalanAndrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculate, J. Exp. Ther. Oncol., 3 (3) (2003), pp. 147-158
[28] Mussard E, Cesaro A, Lespessailles E, Legrain B, Berteina-Raboin S, Toumi H. Andrographolide, a Natural Antioxidant: An Update. Antioxidants (Basel). 2019 Nov 20;8(12):571. doi: 10.3390/antiox8120571. PMID: 31756965; PMCID: PMC6943416.
[29] Rajagopal K, Varakumar P, Baliwada A, Byran G. Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach. Futur J Pharm Sci. 2020;6(1):104. doi: 10.1186/s43094-020-00126-x. Epub 2020 Oct 16. PMID: 33215042; PMCID: PMC7562761.
[30] Mills S and Bone K. Principles and Practices of Phytotherapy. London, New York; Churchill
Livingstone, 2001.
[31] S. Gupta, K.P. Mishra, L. GanjuBroad-spectrum antiviral properties of andrographolide, Arch. Virol., 162 (3) (2017), pp. 611-623 Mar
[32] Gonzalez PA et al. “Andrographolide interferes with T cell activation and reduces experimental autoimmune encephalomyelitis in the mouse.” J Pharmacol Exp Ther. 312, 1:366-72, 2005.
[33] Liu J, Wang ZT, Ji LL. In vivo and in vitro anti-inflammatory activities of neoandrographolide. Am J Chin Med. 2007;35(2):317-28.
[34] [34] Lin TP, Chen SY, Duh PD, Chang LK, Liu YN. Inhibition of the epstein-barr virus lytic cycle by andrographolide. Biol Pharm Bull. 2008 Nov;31(11):2018-23. doi: 10.1248/bpb.31.2018. PMID: 18981566.
[35] V. Pooladanda, S. Thatikonda and C. Godugu, The current understanding and potential therapeutic options to combat COVID-19, Life Sciences (2020), https://doi.org/10.1016/j.lfs.2020.117765
[36] Shi TH, Huang YL, Chen CC, Pi WC, Hsu YL, Lo LC, Chen WY, Fu SL, Lin CH. Andrographolide and its fluorescent derivative inhibit the main proteases of 2019-nCoV and SARS-CoV through covalent linkage. Biochem Biophys Res Commun. 2020 Dec 10;533(3):467-473. doi: 10.1016/j.bbrc.2020.08.086. Epub 2020 Aug 25. PMID: 32977949; PMCID: PMC7447262.
[37] Sukardiman, Ervina M, Fadhil Pratama MR, Poerwono H, Siswodihardjo S. The coronavirus disease 2019 main protease inhibitor from Andrographis paniculata (Burm. f) Ness. J Adv Pharm Technol Res. 2020 Oct-Dec;11(4):157-162. doi: 10.4103/japtr.JAPTR_84_20. Epub 2020 Oct 10. PMID: 33425697; PMCID: PMC7784943.
[38] P. Sagadevan1, S.N. Suresh, R. Ranjithkumar, S. Rathishkumar, S. Sathish, B. Chandarshekar, Traditional use of Andrographis paniculata: Review and Perspectives, International Journal of Biosciences and Nanosciences, Volume 2 (5), 2015, pp.123-131.
[39] A., Philip, K. and Muniandy, S. (2010). Antioxidant potential and content of Phenolic compounds in
ethanolic extracts of selected parts of Andrographis paniculata. Journal of Medicinal Plants Research, 4(3), 197-202,
[40] Chua LS. Review on Liver Inflammation and Antiinflammatory Activity of Andrographis paniculata for Hepatoprotection. Phytother Res. 2014 Jul 10. doi: 10.1002/ptr.5193.
[41] Trivedi NP, Rawal UM, Patel BP. Hepatoprotective effect of andrographolide against hexachlorocyclohexane-induced oxidative injury. Integr Cancer Ther. 2007 Sep;6(3):271-80.
[42] TanapornKhamphayaaPiyachatChanselabPawineePiyachaturawatcApichartSuksamrarndMichael H.NathansoneJittimaWeerachayaphorn Effects of andrographolide on intrahepatic cholestasis induced by alpha-naphthylisothiocyanate in rats, European Journal of Pharmacology, Volume 789, 15 October 2016, Pages 254-264
[43] Chua LS. Review on liver inflammation and antiinflammatory activity of Andrographis paniculata for hepatoprotection. Phytother Res. 2014 Nov;28(11):1589-98. doi: 10.1002/ptr.5193. Epub 2014 Jul 10. PMID: 25043965.
[44] Woo AY, Waye MM, Tsui SK, Yeung ST, Cheng CH. Andrographolide Up-regulates Cellular Reduced Glutathione Level and Protects Cardiomyocytes against Hypoxia/Reoxygenation Injury. J Pharmacol Exp Ther. 2008 Jan 3.
[45] Thisoda P, Rangkadilok N, Pholphana N, Worasuttayangkurn L, Ruchirawat S, Satayavivad J. Inhibitory effect of Andrographis paniculata extract and its active diterpenoids on platelet aggregation. Eur J Pharmacol. 2006 Dec 28;553(1-3):39-45. Epub 2006 Sep 30.
[46] Amroyan E, Gabrielian E, Panossian A, et al. Inhibitory effect of andrographolide from Andrographis paniculata on PAF-induced platelet aggregation. Phytomedicine 1999;6(1):27-31.
[47] Wu TS, Chern HJ, Damu AG, Kuo PC, Su CR, Lee EJ, Teng CM. Flavonoids and ent-labdane diterpenoids from Andrographis paniculata and their antiplatelet aggregatory and vasorelaxing effects. J Asian Nat Prod Res. 2008 Jan;10(1):17-24.
[48] Chen JH, Hsiao G, Lee AR, Wu CC, Yen MH. Andrographolide suppresses endothelial cell apoptosis via activation of phosphatidyl inositol-3-kinase/Akt pathway. Biochem Pharmacol. 2004 Apr 1;67(7):1337-45.
[49] Xu Y, Wei H, Wang J, Wang W, Gao J. Synthesis of andrographolide analogues and their neuroprotection and neurite outgrowth-promoting activities. Bioorg Med Chem. 2019 Apr 16. pii: S0968-0896(19)30042-2. doi: 10.1016/j.bmc.2019.04.025.
[50] Sheeja K, Kuttan G. Activation of cytotoxic T lymphocyte responses and attenuation of tumor growth in vivo by Andrographis paniculata extract and andrographolide. Immunopharmacol Immunotoxicol. 2007;29(1):81-93.
[51] Xu Y, Chen A, Fry S, Barrow RA, Marshall RL, Mukkur TK. Modulation of immune response in mice immunised with an inactivated Salmonella vaccine and gavaged with Andrographis paniculata extract or andrographolide. Int Immunopharmacol. 2007 Apr;7(4):515-23. Epub 2007 Jan 17.
[52] Wang HC, Tsay HS, Shih HN, Chen YA, Chang KM, Agrawal DC, Huang S, Lin YL, Lee MJ. Andrographolide relieved pathological pain generated by spared nerve injury model in mice. Pharm Biol. 2018 Dec;56(1):124-131. doi: 10.1080/13880209.2018.1426614.
[53] Mussard E, Cesaro A, Lespessailles E, Legrain B, Berteina-Raboin S, Toumi H. Andrographolide, a Natural Antioxidant: An Update. Antioxidants (Basel). 2019;8(12):571. Published 2019 Nov 20. doi:10.3390/antiox8120571
[54] William J Sandborn, Stephan R Targan, Vera S Byers, Dean A Rutty, Hua Mu, Xun Zhang, Tom Tang, Andrographis paniculata Extract (HMPL-004) for Active Ulcerative Colitis Am J Gastroenterol. 2013 January; 108(1): 90–98. Published online 2012 October 9. doi: 10.1038/ajg.2012.340
[55] Andrographis, Materia Medica, https://materiamedicaresource.wordpress.com/2013/04/05/andrographis/, retrieved 02/15/2020
[56] Wang S, Hu Y, Tan W, Wu X, Chen R, Cao J, Chen M, Wang Y. Compatibility art of traditional Chinese medicine: from the perspective of herb pairs. J Ethnopharmacol. 2012 Sep 28; 143(2):412-23.
[57] Bennouar S, Cherif AB, Kessira A, Bennouar DE, Abdi S. Vitamin D Deficiency and Low Serum Calcium as Predictors of Poor Prognosis in Patients with Severe COVID-19. J Am Coll Nutr. 2021 Jan 12:1-11. doi: 10.1080/07315724.2020.1856013. Epub ahead of print. PMID: 33434117.
[58] Munshi R, Hussein MH, Toraih EA, Elshazli RM, Jardak C, Sultana N, Youssef MR, Omar M, Attia AS, Fawzy MS, Killackey M, Kandil E, Duchesne J. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol. 2021 Feb;93(2):733-740. doi: 10.1002/jmv.26360.
[59] Sethuram R, Bai D, Abu-Soud HM. Potential Role of Zinc in the COVID-19 Disease Process and its Probable Impact on Reproduction. Reprod Sci. 2021 Jan 7:1–6. doi: 10.1007/s43032-020-00400-6. Epub ahead of print. PMID: 33415646; PMCID: PMC7790357.
[60] Mlcek J, Jurikova T, Skrovankova S, Sochor J. Quercetin and Its Anti-Allergic Immune Response. Molecules. 2016 May 12;21(5):623. doi: 10.3390/molecules21050623. PMID: 27187333; PMCID: PMC6273625.
[61] Weng Z, Zhang B, Asadi S, Sismanopoulos N, Butcher A, Fu X, Katsarou-Katsari A, Antoniou C, Theoharides TC. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS One. 2012;7(3):e33805. doi: 10.1371/journal.pone.0033805. Epub 2012 Mar 28. PMID: 22470478; PMCID: PMC3314669.
[62] Gröber U, Holick MF. The coronavirus disease (COVID-19) – A supportive approach with selected micronutrients. Int J Vitam Nutr Res. 2021 Jan 25:1-22. doi: 10.1024/0300-9831/a000693. Epub ahead of print. PMID: 33487035.
[63] Majeed M, Nagabhushanam K, Gowda S, Mundkur L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition. 2021 Feb;82:111053. doi: 10.1016/j.nut.2020.111053. Epub 2020 Nov 11. PMID: 33321395; PMCID: PMC7657009.
[64] Jinsong Zhang, Ramy Saad, Ethan Will Taylor, Margaret P. Rayman, Selenium and selenoproteins in viral infection with potential relevance to COVID-19, Redox Biology, Volume 37, 2020,101715, ISSN 2213-2317, https://doi.org/10.1016/j.redox.2020.101715.

A monumental survey, Donnie correlating traditional treatment of this global threat with conventional medicine. Thanks for this illuminating effort. While we all have our own choices to make in terms of vaccination, no-one should turn from the benefits of natural medicine, which can be used as a vaccine adjuvant. And still is my own first choice for virus prevention, together with life style measures, of course such as mask wearing, etc. Prevention will always be key.