The mRNA vaccines made by Pfizer and Moderna have been heavily promoted for ending the Covid-19 pandemic. With claims that they are ‘extraordinary’ and ‘revolutionary,’ these vaccines have occupied center stage for more than a year.
The CDC is not accounting for COVID-19 risk factors or natural immunity. It is hard to believe that the risk benefit balance favors a 15-year-old young man who has recovered from COVID-19, and who has detectable antibodies, getting two doses of an mRNA vaccine. Such an individual is accepting a non-negligible risk of myocarditis, with limited upside in terms of decreased risk of severe infection.[1]
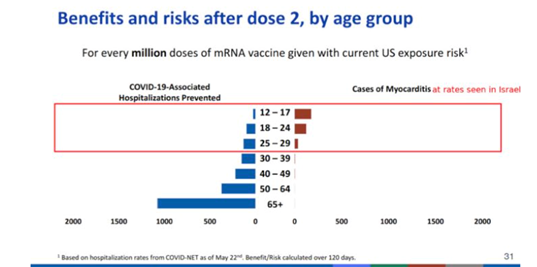
A recently published retrospective case series studied patients within the US Military Health System who experienced myocarditis after a COVID-19 vaccination between January and April 2021. The study found that myocarditis occurred in previously healthy military patients with similar clinical presentations following receipt of an mRNA COVID-19 vaccine. A total of 23 male patients presented with acute onset of marked chest pain within 4 days after receipt of an mRNA COVID-19 vaccine. The military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period. While the observed number of myocarditis cases was small, the number was higher than expected among male military members after a second vaccine dose.[2]
But wait, there are other, potentially safer and equally effective vaccines now available. When the Maryland-based biotech firm Novavax announced its latest trial results last week—with an efficacy rate of more than 90 percent even against coronavirus variants—the news barely made headlines.
I suspect we are all suffering from ‘vaccine information overload.’ But the Novavax vaccine deserves serious consideration. The approach used by Novavax was first implemented for the hepatitis B vaccine, which has been used in the U.S. since 1986. The pertussis vaccine, a standard childhood vaccine, is also made in this way. Because it is based on older, more familiar science, people who have been wary of getting the mRNA vaccines may find Novavax an acceptable and appealing alternative.
Novavax Protein-based Vaccine
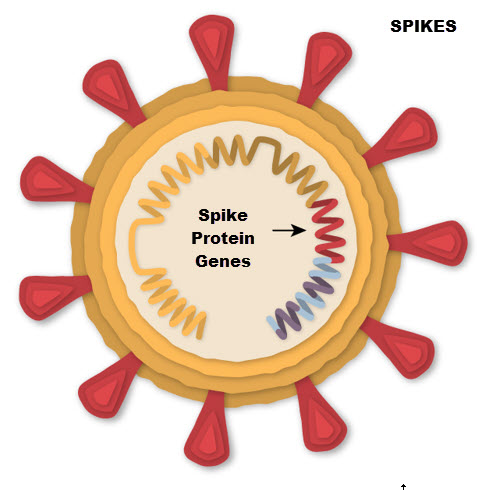
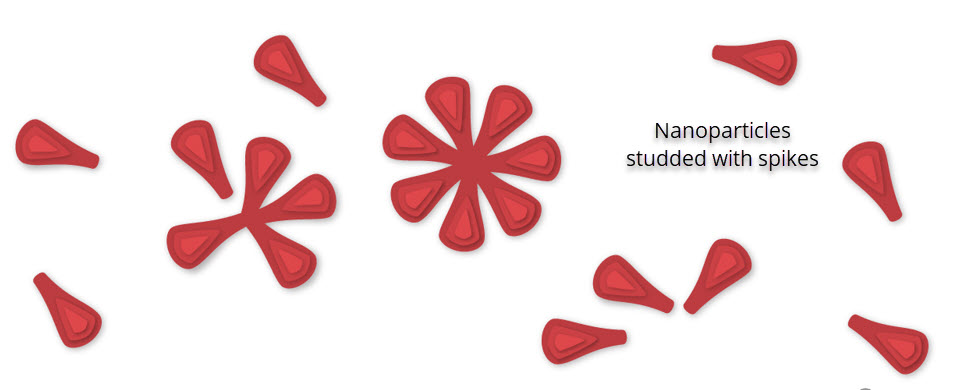
Here’s how the Novavax vaccine works: The SARS-CoV-2 spike (S) glycoprotein is a major component of the virus envelope, and is essential for receptor binding, fusion, virus entry, and a target of host immune defense. The Novavax vaccine (called NVX-CoV2373) is made with lab-grown copies of that protein (made from moth cells) and works by teaching the immune system to make antibodies to the spike protein. Spike proteins are harvested from the moth cells and assembled into nanoparticles. While the nanoparticles mimic the molecular structure of the coronavirus, they cannot replicate or cause COVID-19.[3]
The other vaccines now widely used use genetic instructions for the body to make its own spike protein. The Novavax vaccine contains proteins and no genetic material. This makes it very similar to a protein-based influenza vaccine.
Results from the Phase 3 trial on Novavax, which enrolled nearly 30,000 participants across 119 sites in the United States and Mexico, show the recombinant protein-based vaccine provided 100% protection against moderate and severe cases of COVID-19, with an overall efficacy of 90.4%.[4]
The inner bark of Quillaja saponaria, the Chilean soap bark tree, plays a central role in the Novavax vaccine. It’s added to the vaccine as an adjuvant to boost the immune response, creating higher levels of antibodies. Quillaia is used as traditional medicine for the common cold, cough, bronchitis, high cholesterol, and many other conditions. As an herbal medicine, the saponin content of the bark helps thin mucus in the respiratory tract, facilitating the removal of phlegm through coughing. Most important in the context of vaccine development, the bark contains saponins that boost the body’s reaction to vaccines.
Quillaia has a good safety profile. In addition to being used as an herbal medicine, quillaia is added to frozen dairy desserts, candy, baked goods, and as a foaming agent in root beer.[5]

When pulverized and soaked in water, the bark is transformed into a brown, bitter, bubbly fluid. This soapy and gooey plant material becomes the raw material for one of the world’s most coveted vaccine adjuvants: QS-21. Adjuvants are compounds that boost the body’s immune reaction to a vaccine. Because of their potential risks to human health, however, only a handful of adjuvants have been approved by the U.S. Food and Drug Administration, and QS-21 is one of the newest.
A single gram of powdered QS-21 costs more than $100,000, though only about $5 worth is needed for each shot. Nine years ago, researchers estimated that the global supply of pharmaceutical-grade Quillaja extract was sufficient for just 6 million doses of vaccine. Despite challenges in production, quality control, stability and toxicity, the QS-21 fraction from this extract has exhibited exceptional adjuvant properties for a range of antigens. It possesses an ability to augment clinically significant antibody and T-cell responses to vaccine antigens against a variety of infectious diseases, degenerative disorders and cancers.[6]
One of the problems is overharvesting, because the Chilean soap bark tree is somewhat rare. “If you take out all the trees in one shot and deplete the source of saponin, you are in deep sh*t in the future,” says Garo Armen, whose company, Agenus, helped bring QS-21 to market. Novavax has its own saponin-based adjuvant, called Matrix-M. Co-formulation with Matrix-M has shown to significantly increase vaccine immunogenicity, resulting in antigen-specific humoral and cellular immune responses.[7]
As an alternative, researchers have been studying other species that contain soapbark saponins and their performance as vaccine adjuvants, which have shown to trigger humoral and cellular immune responses comparable to saponins from Chilean soap bark. Leaf saponins of soapbark can also enhance long-term specific immune responses and promote dose-sparing effects in experimental vaccines.[8]
The Novavax vaccine can be stored in standard refrigerators, making it easier to distribute. It also appears to be 90% effective against variants. In an ongoing randomized, placebo-controlled, phase 1/2 trial in healthy adults, NVX-CoV2373, administered in a two-dose regimen 21 days apart, had an acceptable safety profile, was associated with a strong, Th1-biased, antigen-specific polyfunctional CD4+ T-cell response, and induced neutralizing antibody responses 4-fold higher than levels in convalescent sera from predominantly moderate to severe Covid-19 cases.[9]
Vaxxinity Peptide-based Vaccine
Another vaccine that shows promise as an alternative to mRNA vaccines is the peptide-based vaccine created by Vaxxinity. Prior to Covid-19, Vaxxinity’s focus was on developing a vaccine for Alzheimer’s, using an innovative approach to treating the devastating disease.[10] Vaxxinity currently has a commercial antibody test available under the US FDA emergency-use authorization.
The Vaxxinity Covid-19 vaccine, known as UB-612, is the first multitope peptide-based vaccine for COVID-19. It activates both an antibody response and a T-cell response to SARS-CoV-2 for robust protection.[11]
Of multiple SARS-CoV-2 vaccines currently in clinical development, almost all focus solely on the spike protein. UB-612 is designed to target a critical antigen from the spike protein, the Receptor Binding Domain, thought to be necessary for viral attachment to human cells, plus additional viral epitopes (from other viral structural proteins) designed to promote B-cell and CD8+ T-cell memory responses.[12]
Because Vaxxinity vaccines are made from synthetic peptides, they can swap target epitopes and amino acid sequences with relative ease. This offers the potential to respond rapidly as needed to changes in the SARS-CoV-2 virus. The vaccine was designed to induce a broad immune response, and in laboratory studies demonstrated the ability to reduce viral load and prevent COVID-19 infection in a mouse challenge model and in rhesus macaques.[13] The studies showed that UB-612 induced neutralizing antibodies in 100% of participants was well-tolerated and had a reassuring safety profile, and induced antigen-specific polyfunctional CD4+/CD8+ T cell responses.
Mei Mei Hu, the co-founder and CEO of Vaxxinity, stated in a press release: “We have been studying the new South African mutation, and we believe it is a significant threat to public health. As a result, we are not only testing UB-612 against these variants. Still, we have also begun early testing and design of a second vaccine candidate that addresses both the UK and the South African variant and the potential to be a single-dose vaccine for primary immunization.” Subject to regulatory approval, Vaxxinity plans to supply up to 500 million vaccine doses globally in 2021.[14]
Vaxxinity has signed a purchase order with the government of Paraguay for 1 million doses of Vaxxinity’s UB-612 COVID-19 vaccine.[15] Results of a robust clinical trial should be available by September,[16] which means UB-612 may get early approval in the United States by the fall of 2021.
Making Sense of the Vaccines
Currently, the national supply of vaccines for Covid-19 exceeds the demand (this includes the Pfizer, Moderna, and Johnson & Johnson vaccines). This creates a problem for the availability of new, potentially better vaccines. If the FDA deems there is no urgency to bring a new vaccine to market, the Novavax vaccine might not be available in the U.S. for months.
Although mRNA vaccines have received enormous press as the most effective and preferred vaccines for Covid-19, this may not actually be the case. The Pfizer and Moderna vaccines were the first to complete Phase 3 clinical trials and thus were granted emergency usage by the FDA. But these vaccines are more expensive to manufacture, and distribution is more complicated than for traditional types of vaccines. Most concerning is that the side effects for both Pfizer and Moderna are more common and more severe than with traditional types of vaccines.
References
[1] Vinay Prasad, MD, MPH, Ramin Farzaneh-Far, MD, Wes Pegden, PhD, Venk Murthy, MD, PhD, Amy Beck, MD, MPH June 29, 2021, CDC’s All-or-Nothing Approach to Teen COVID Vaccination Is All Wrong Medscape Today, https://www.medpagetoday.com/opinion/second-opinions/93340?xid=nl_vanayprasad_2021-06-29&eun=g1065123d0r&utm_source=Sailthru&utm_medium=email&utm_campaign=VinayPrasad_062921&utm_term=NL_Gen_Int_Vinay_AYWDRL_Large_Active
[2] Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. Published online June 29, 2021. doi:10.1001/jamacardio.2021.2833
[3] Jonathan Corum and Carl Zimmer, How the Novavax Covid-19 Vaccine Works – The New York Times, Updated May 7, 2021, retrieved June 16, 2021
[4] https://ir.novavax.com/news-releases/news-release-details/novavax-announces-positive-preclinical-data-combination, retrieved June 22, 2021
[5] https://en.wikipedia.org/wiki/Quillaja_saponaria
[6] Ragupathi G, Gardner JR, Livingston PO, Gin DY. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev Vaccines. 2011;10(4):463-470. doi:10.1586/erv.11.18
[7] Magnusson SE, Altenburg AF, Bengtsson KL, et al. Matrix-M™ adjuvant enhances immunogenicity of both protein- and modified vaccinia virus Ankara-based influenza vaccines in mice. Immunol Res. 2018;66(2):224-233. doi:10.1007/s12026-018-8991-x
[8] Cibulski S, Rivera-Patron M, Suárez N, Pirez M, Rossi S, Yendo AC, de Costa F, Gosmann G, Fett-Neto A, Roehe PM, Silveira F. Leaf saponins of Quillaja brasiliensis enhance long-term specific immune responses and promote dose-sparing effect in BVDV experimental vaccines. Vaccine. 2018 Jan 2;36(1):55-65. doi: 10.1016/j.vaccine.2017.11.030. Epub 2017 Nov 23. PMID: 29174676.
[9] Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, Lalloo U, Masilela MSL, Moodley D, Hanley S, Fouche L, Louw C, Tameris M, Singh N, Goga A, Dheda K, Grobbelaar C, Kruger G, Carrim-Ganey N, Baillie V, de Oliveira T, Lombard Koen A, Lombaard JJ, Mngqibisa R, Bhorat AE, Benadé G, Lalloo N, Pitsi A, Vollgraaff PL, Luabeya A, Esmail A, Petrick FG, Oommen-Jose A, Foulkes S, Ahmed K, Thombrayil A, Fries L, Cloney-Clark S, Zhu M, Bennett C, Albert G, Faust E, Plested JS, Robertson A, Neal S, Cho I, Glenn GM, Dubovsky F, Madhi SA; 2019nCoV-501 Study Group. Efficacy of NVX-CoV2373 Covid-19 Vaccine against the B.1.351 Variant. N Engl J Med. 2021 May 20;384(20):1899-1909. doi: 10.1056/NEJMoa2103055. Epub 2021 May 5. PMID: 33951374; PMCID: PMC8091623.
[10] https://www.clinicaltrialsarena.com/analysis/vaxxinity-ceo-mei-mei-hu-on-vaccine-research-and-democratising-health/?utm_source=Army%20Technology&utm_medium=website&utm_campaign=Must%20Read&utm_content=Image, retrieved June 22, 2021
[11] bioRxiv: A Novel SARS-CoV-2 Multitope Protein/Peptide Vaccine Candidate is Highly Immunogenic and Prevents Lung Infection in an Adeno Associated Virus Human Angiotensin-Converting Enzyme 2 (AAV hACE2) Mouse Model
[12] https://vaxxinity.com/covid-19-vaccine/, retrieved June 21, 2021
[13] News Medical: Novel “multitope” vaccine shows promise in the fight against COVID-19 [Link]
[14] https://www.precisionvaccinations.com/vaccines/ub-612-covid-19-vaccine, June 22, 2021
[15] https://www.businesswire.com/news/home/20210621005250/en/Vaxxinity-Signs-Purchase-Order-with-the-Government-of-Paraguay-for-1-Million-Doses-of-Vaxxinity%E2%80%99s-UB-612-COVID-19-Vaccine, retrieved June 22, 2021
[16] https://clinicaltrials.gov/ct2/show/NCT04545749, retrieved June 22, 2021

Any opinion about the attenuated Covaxin vaccine?
Certainly would have been good to understand this when I took the Moderna vaccine. First dose, there was no problem at all. Second dose, another story altogether. Within an hour of getting vaccinated I developed acute Tinnitius + audio hypersensitivity to low vibrations and began itching all over my body although there was no rash. This lasted 24-7 for about 2 months, after which the tinnitus remains in a milder version and there is occasional itching if I have not had a good night’s rest or am over-stressed.
This information and your knowledge seem quite substantial, and well-researched.
Thank you for taking a strong position, although I would assume it is not popular amongst many professionals, as the need to find a solution has been paramount – thus, waiting for further research prior to getting these vaccines out didn’t seem an option.
As one who suffered adverse affects – I took the position as happy to be vaccinated and wanting to add my story to the findings for Moderna in further development of their vaccine. I provided medical history and spoke with them on two occasions at length. My hope is that they use this information and don’t bury it – glossing over the multitude of side effects people may suffer in getting vaccinated.
I doubt I would opt for a Moderna booster – and would hope if this is needed to find a safer option for that if it is necessary.
Donnie, reading Mary Jo’s comment leads me to add mine. The autoimmune reality is a worry. For a person with Hashimoto’s, Celiac Disease, tinnitus, chronic itching w/o lesions, and reading that persons with Hashi’s have reacted to the vaccines (which ones I do not know). plus the issue of cause and effect in addition to existing autoimmune diseases, will these two new vaccines be any less risk for autoimmune reactions, short or long term?
Dear Donnie,
I noticed you wrote an article on Covavax and Novavax. My understanding is that they are the same. Novavax launched its vaccine, in India, calling it Covavax.
Whether under Novavax, or Covavax, I wish we had access to them in the US. As someone who had terrible long-hual Covid (over 1 year), I definitely don’t want it again. I have no doubt my healthy life-style and using plant medicine, including your products, and TCM, helped me turn the corner. I barely made it out alive and it was not without its consequences. I would be grateful for a safer vaccine in addition to my ongoing pursuit of better health.