By Donnie Yance
Questioning Conventional Screening Approaches
According to the United States Preventive Services Task Force (USPSTF) analysis of two major randomized clinical trials, routine Prostate-specific antigen (PSA) screening prevents approximately one prostate cancer-related death per 1,000 men screened.¹ This sobering statistic challenges the historical perception of PSA screening as an essential preventive measure. Moreover, recent studies suggest that the psychological burden and financial costs of widespread PSA screening may outweigh its limited mortality benefits. Many of these biopsies turn out to be unnecessary, causing anxiety and discomfort for patients that in some cases plagues a man for the rest of his life.
I am not of the opinion that men should not test their PSA. However, I do believe PSA testing should include more comprehensive testing methods, including PSA total and free percentage, along with several new urine tests that are considered even more accurate than a biopsy. Also keep in mind that healthy PSA ranges differ for each man. In other words, my healthy range may be different from your healthy range, and prostate enlargement and prostatitis both can cause elevated PSAs.
Shifting Treatment Paradigms
The aggressive treatment paradigm—typically involving radical prostatectomy, radiation therapy, or androgen deprivation therapy—has come under scrutiny. Research indicates that a significant proportion of prostate cancers are indolent (cancers that progress and grow slowly).
The landmark 15-year ProtecT trial² provided compelling evidence of this, randomizing men with clinically localized prostate cancer between prostatectomy, external-beam radiation therapy, and monitoring. Among 2,664 eligible participants, 1,643 (62%) accepted randomization. Despite substantial crossover between treatment groups, with approximately 60% of monitoring patients eventually receiving interventional treatment, the study found no significant difference in overall survival between the groups after 15 years of follow-up. These findings strongly suggest that immediate aggressive treatment may not always be necessary or beneficial.
Evidence-Based Treatment Selection
Research from a comprehensive 2012 longitudinal study³ demonstrated that for men with low-risk localized prostate cancer who have a life expectancy under 10 years, watchful waiting emerges as the optimal treatment strategy. However, for patients with low or intermediate-risk localized prostate cancer and a life expectancy exceeding 10 years, clinical evidence from 2012 remains inconclusive regarding the superiority of available treatments: watchful waiting, radical prostatectomy, external beam radiation therapy, or brachytherapy (a type of internal radiation therapy that involves placing radioactive seeds or wires directly into the tumor site).
Quality of Life Considerations
An often overlooked aspect of prostate cancer treatment is its impact on quality of life. Treatment-related side effects can include urinary incontinence, erectile dysfunction, and bowel problems. These complications must be carefully weighed against the potential benefits of intervention, particularly in cases of low-risk disease where active surveillance might be equally effective in terms of survival outcomes.
Biopsies Might Promote the Spread of Cancer
Doctors Mark Goldstein and Luca Mascitelli suggest the small benefit might be due to the biopsy process itself. They note that prostate biopsies might actually help cancer cells spread, similar to what’s seen in breast cancer when there’s a long delay between biopsy and surgery.
Challenges with biopsies include:
- Biopsy needles can damage tissue, activating angiogenesis
- The procedure creates inflammation that may suppress the immune system⁴
- Increased risk of infection⁵
- Prostate biopsies take more tissue samples than breast biopsies
- Some patients have a series of multiple biopsies taken for monitoring
- Evidence of cancer spread after biopsy
- Cancer cells appear in blood immediately after prostate biopsy. In one study of 569 patients: 70% had cancer cells in bone marrow before surgery, 57% still had these cells 20 months after surgery. These patients had 7 times higher risk of the cancer returning ⁶
PSA Testing: Benefits and Limitations
PSA testing alone may do more harm than good, but not if you combine it with other testing modalities.
Richard Ablin, the inventor of the PSA test, has become a vocal critic of its widespread use as a diagnostic tool for prostate cancer. He argues that “routine PSA screening does far more harm to men than good” and that the medical industry involved in prostate screening and treatment has “maimed millions of American men.”
Key issues with the PSA test include:
- A 78% false positive rate
- Elevated PSA levels can be caused by factors other than cancer
- The test can lead to unnecessary biopsies and harmful surgical procedures
A recent 2024 JAMA study⁷ found that PSA screening alone compared with standard practice without routine screening reduced prostate cancer deaths at a median follow-up of 15 years. However, the absolute reduction in deaths was small and non-significant.
Dr. Mark Scholz, executive director of the Prostate Cancer Research Institute (PCRI), criticizes “the urology world’s persistent overtreatment mindset,” noting that treatments like radiation and surgery reduce mortality by only 1%-2% in men with low and intermediate-risk disease, and by less than 10% in men with high-risk disease. ‘Not all cancer needs to be cured.’
“We no longer have a one-size-fits-all approach,” says Dr. Patrick Pilié of The MD Anderson Cancer Center.
Watchful waiting is significantly associated with a low risk of prostate cancer progression and mortality among men with less than 10 years of life expectancy.⁸
A more nuanced, personalized approach to prostate cancer screening and management is emerging. Rather than adhering to universal screening guidelines, contemporary best practices advocate for individualized risk assessment and targeted intervention strategies.⁹
Biomarkers in Prostate Cancer
Biomarkers play a crucial role in the clinical management of prostate cancer, serving as valuable tools that can be used for diagnosis, prognosis, and prediction to facilitate patient-specific treatment approaches. These molecular indicators, which can be measured in blood, urine, or tissue samples, provide objective information about biological processes, pathological conditions, or responses to therapeutic interventions.
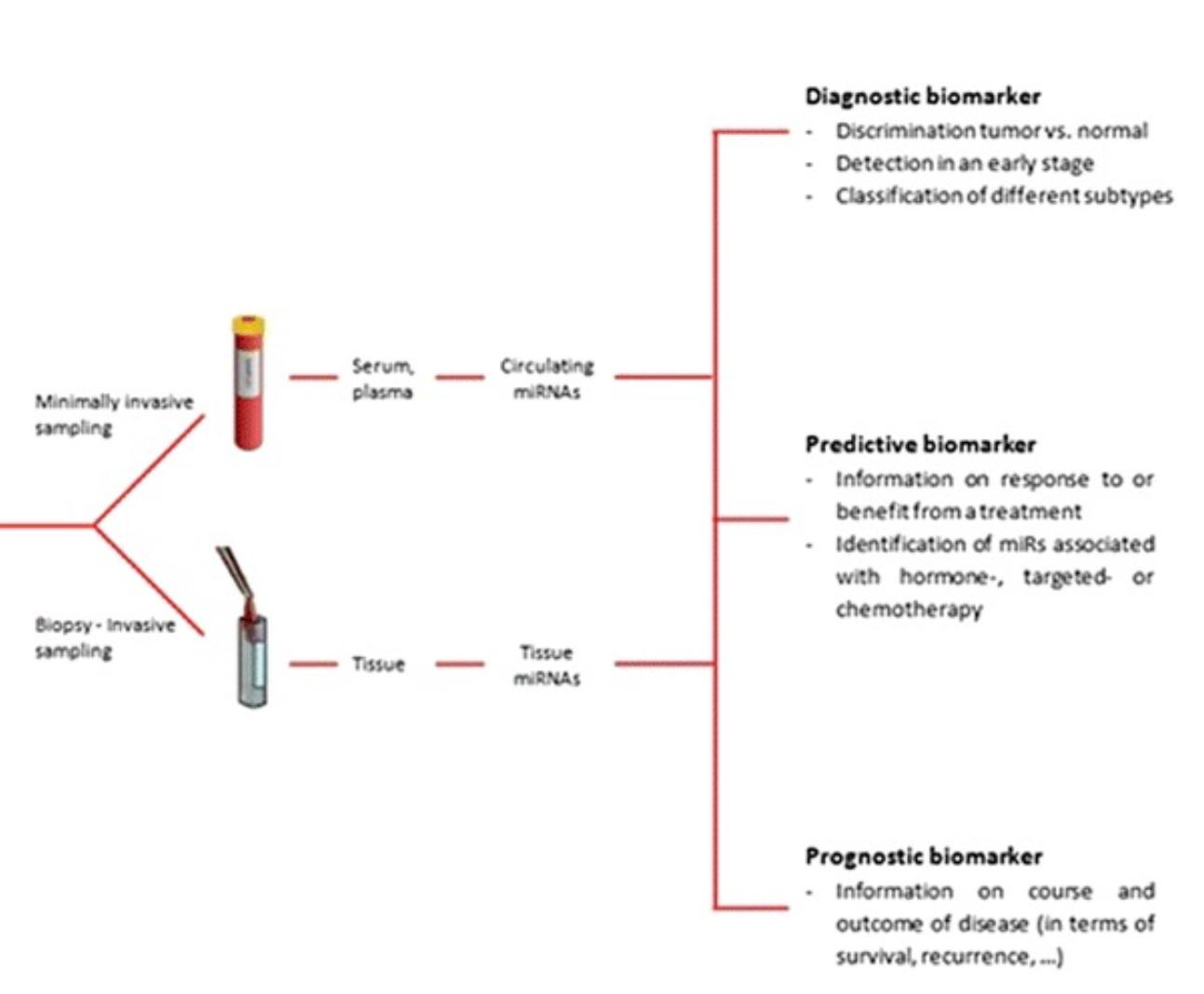
In the context of prostate cancer, biomarkers have revolutionized clinical practice by enabling earlier detection, more accurate risk stratification, and better-informed treatment decisions. From the well-established PSA to emerging genomic and proteomic markers, the landscape of prostate cancer biomarkers continues to evolve rapidly with advances in molecular biology and analytical technologies.
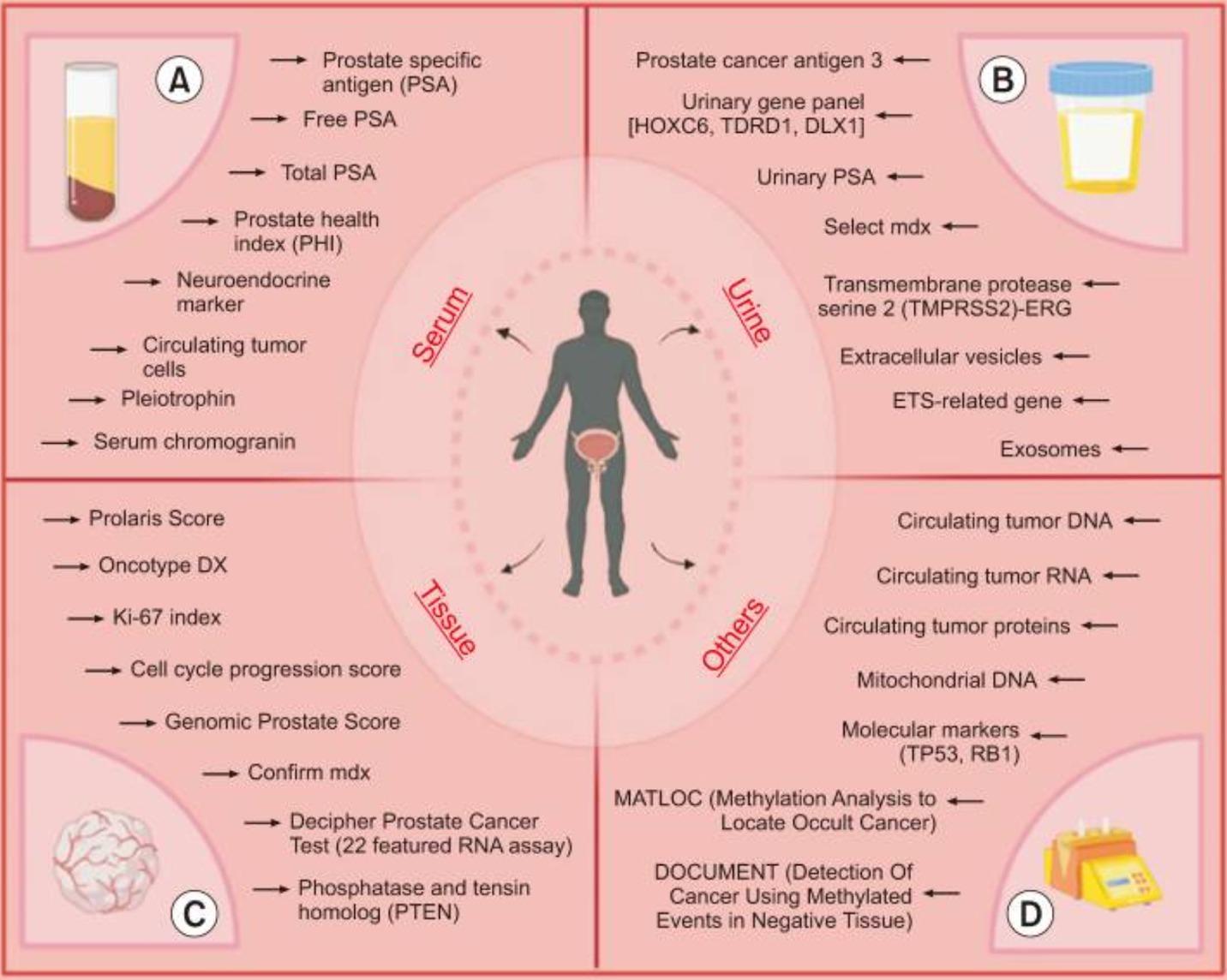
Classification of biomarkers in the detection of prostate cancer using different samples. ERG, ETS-related gene; ETS, E26 transformation-specific.
This comprehensive overview explores the current state of biomarkers in prostate cancer management, their clinical applications, limitations, and the promising future directions that may further enhance personalized medicine approaches for patients with this heterogeneous disease.
Better Ways to Understand PSA
While PSA testing remains a valuable diagnostic tool, its application requires careful consideration within a broader clinical context.
Critical factors include:
- PSA velocity (rate of change over time)
- Percent free PSA (values >25% associated with 5-9% cancer risk)¹⁰
Three key principles should guide our approach to prostate cancer screening and management:
- Prostate cancer must be understood as a systemic condition rather than merely a localized disease process ¹¹
- The typically indolent nature of prostate cancer—often progressing over decades if at all—suggests that preventive lifestyle modifications and specific herbal and nutritional supplementation may offer greater benefit than aggressive screening protocols
- While PSA testing remains a useful diagnostic tool, its application should be guided by comprehensive clinical assessment rather than rigid screening guidelines. What’s important is to establish a baseline and graph the movement. PSA is not meaningless, but its behavior in relation to cancer varies from one patient to another.
Advanced Assessment Options
PSA Doubling Time
The longer the doubling time, the slower the cancer is growing.¹² ¹³
Circulating Tumor Cells (CTCs)
CTCs are now considered a strong tool to understand the molecular characteristics of prostate cancer, and to be used and analyzed as a ‘liquid biopsy’ in the attempt to grasp the biological portrait of the disease in the individual patient.¹⁴
Liquid biopsy testing can not only provide added benefit in prostate cancer diagnosis, but also give insights to the potential aggressiveness of the cancer and various biomarkers that can better predict the ideal course of treatment.
Prostate Health Index (PHI)
PHI is a sophisticated blood test that offers several advantages over standard PSA testing, by using a formula that combines all 3 PSA forms (total PSA, free PSA, and p2PSA) into a single score.¹⁵
A Johns Hopkins-led study demonstrated that:
- PHI outperformed traditional PSA testing
- Showed excellent results in identifying clinically significant cancers
- Achieved optimal results when combined with MRI
- Reduced unnecessary biopsies without missing significant cancers
4K (4-Kallikrein panel) Score
This advanced blood test combines the three forms of PSA with another prostate enzyme called Human kallikrein 2.
- Effectively predicts high-grade prostate cancer risk
- Serves as an initial screening tool for biopsy decisions
- Complements MRI imaging in cancer detection
Urine-Based Tests
Urine presents distinct advantages over other biological specimens for the identification of prognostic and diagnostic biomarkers. The prostate gland secretes various components that are transported to the urinary system and can be detected in urine samples, serving as valuable biological markers for both the detection and prognosis of prostate cancer. Notably, patients with prostate cancer exhibit lower urinary PSA levels compared to men without cancer symptoms, with these levels decreasing progressively as tumor stages advance.
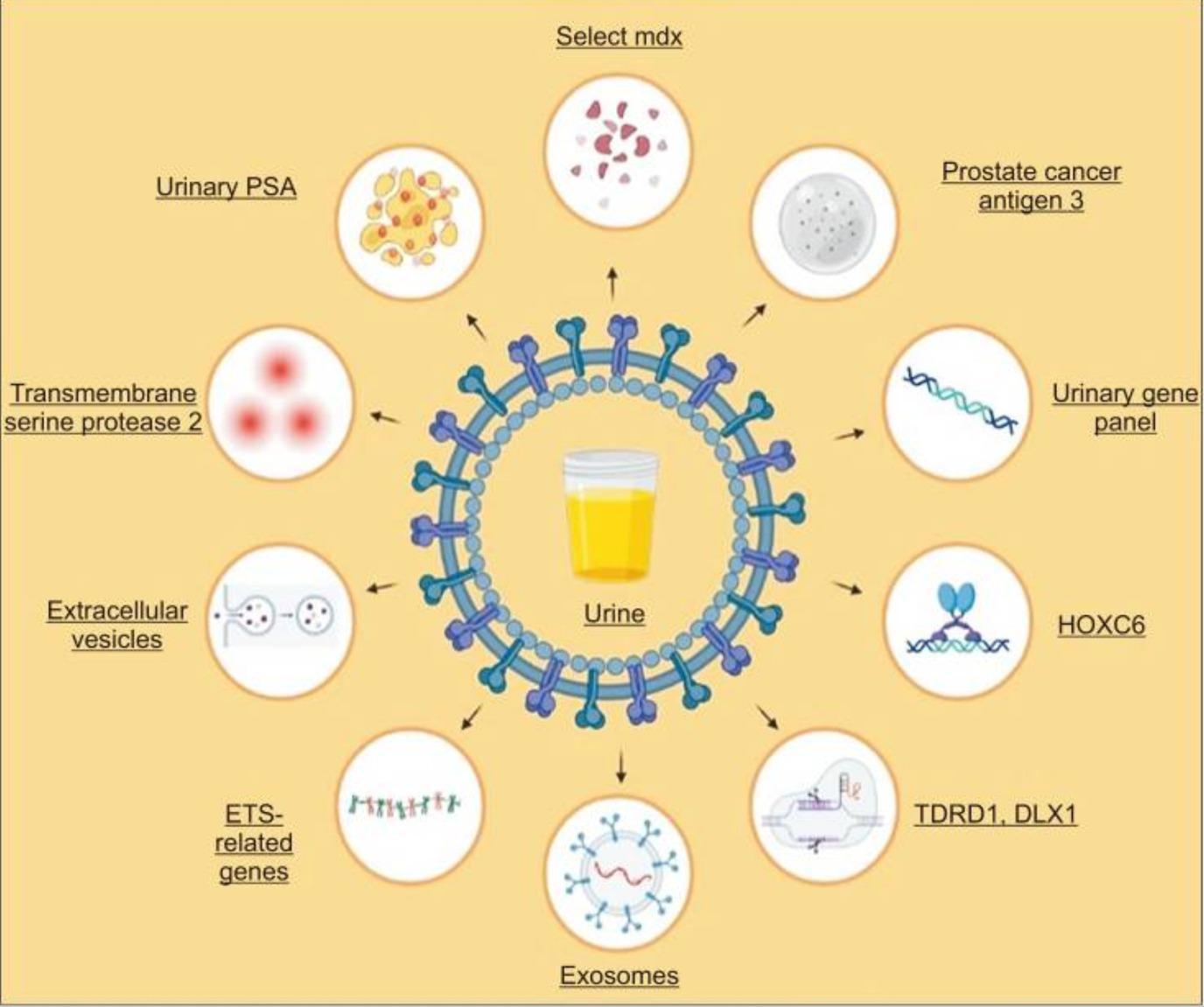
Urine-based biomarkers used in diagnosis of prostate cancer. PSA, prostate specific antigen; ETS, E26 transformation-specific.
Prostate Cancer Antigen 3 (PCA3)
- FDA-approved biomarker test
- Recognized by American Urological Association
- Helps determine biopsy necessity
- Useful for initial and repeat biopsy decisions
When initial findings warrant further investigation, the urinary PCA3 test offers superior specificity. Studies demonstrate PCA3 is overexpressed 66-fold in over 95% of prostate cancer cells compared to normal or benign hyperplastic prostate tissue, providing significantly greater diagnostic accuracy than PSA alone.
The urine is collected following a digital rectal exam (DRE) that causes the prostate to shed whole prostate cells into the urine. These cells can then be analyzed for a certain gene associated with the presence of cancer. Thus, the PCA3 is “a molecular biology assay that measures the expression of PCA3 (prostate cancer gene 3) mRNA (messenger RNA) in urine samples.”
The analysis gives a numeric score, and the cutoff point (“red flag”) for cancer is considered to be 35 or higher. One study even suggested that for men who have never had a prostate biopsy, a score of 60 or higher suggests a high likelihood that a biopsy will find cancer.
SelectMDx Urine Test
- Measures expression of two mRNA biomarkers
- Identifies aggressive cancer cell lines
- Aids in biopsy decision-making
- For more information: SelectMDx for Patients
ExoDx™ Prostate Test
- Advanced urine-based diagnostic
- More information: ExoDx Information
MyProstateScore 2.0 (MPS2) Urine Test
- Specialized prostate cancer screening: 18 Unique Gene Transcripts
MPS2 analyzes 18 unique gene transcripts within models shown to predict clinically significant cancer across a variety of patient groups.
For more information: MyProstate Score
Transmembrane Protease Serine 2-ERG (TMPRSS2-ERG)
- TMPRSS2-ERG gene fusions, the most common molecular subtype of ETS family gene fusions, occur in ~50% of prostate carcinomas (PCa) and ~20% of high-grade prostatic intraepithelial neoplasia intermingled with adjacent PCa demonstrating identical gene fusions
- TMPRSS2-ERG fusion is:
A common recurrent chromosomal aberration in this malignancy
A strong androgen promoter (transmembrane serine protease) and an oncogene
Mi-Prostate Score (MiPS)
- The MiPS test incorporates blood PSA levels and two molecular RNA markers PCA3 and TMPRSS2-ERG fusion specific for prostate cancer in one final score
- For More Information: MLabs (800-862-7284) https://mlabs.umich.edu/
- The blood-based PHI and urinary PCA3 are two US FDA approved biomarkers for diagnosis of PCa
- PHI and PCA3 are able to predict pathological findings on radical prostatectomy specimen, such as tumor volume and Gleason score
- Only PHI could predict seminal vesicle invasion and only PCA3 could predict multifocality
Prostate Magnetic Resonance Imaging (MRI)
A prostate MRI can help reduce unnecessary biopsies. ¹⁶ Since it is radiation free and non-invasive, an MRI scan is a promising alternative to biopsy, especially when combined with the more advanced PSA panels.
Three different kinds of MRI tests for prostate cancer include:
- Contrast-enhanced MRI — uses a dye to make the pictures clearer. A healthcare professional puts the dye into a vein before the scan
- MRI with endorectal coil — MRI with endorectal coil uses a thin wire device inserted in the rectum to get better pictures of the prostate
- Multiparametric MRI — also called mpMRI, tells the healthcare team more about the prostate tissue. This kind of MRI can help show the difference between healthy prostate tissue and prostate cancer ¹⁷
Genetic Factors in Prostate Cancer Risk and Prevention
Research has identified several key genes that, when altered by specific mutations, significantly influence prostate cancer susceptibility. Understanding these genetic factors enables more informed screening decisions and personalized risk assessment strategies.
Genetic testing, also called germline testing, serves multiple purposes beyond simply predicting prostate cancer risk. This testing opens the door to proactive preventive approaches, particularly through herbal, nutritional, and dietary interventions that can influence genetic expression through epigenetic mechanisms.
The power of epigenetics lies in its fundamental truth: our genes are not our destiny. Environmental factors, lifestyle choices, and targeted nutritional interventions can modify how our genes are expressed, potentially reducing cancer risk even in those with genetic predispositions. I explored this concept in depth in my previous blog post, Your Genes Are Not Your Destiny: The Science Of Epigenetics.
Primary Genetic Risk Factors
BRCA1 and BRCA2 Genes
These tumor suppressor genes normally function as cellular guardians, preventing cancerous changes. However, inherited mutations can impair their protective capabilities. While BRCA mutations are most commonly associated with breast and ovarian cancer risk, men who carry these mutations, particularly BRCA2 mutations, face an elevated risk of developing prostate cancer.
DNA Mismatch Repair Genes (MSH2, MSH6, MLH1, PMS2)
These genes maintain genomic stability by correcting DNA replication errors. Inherited mutations in any of these genes cause Lynch syndrome, a hereditary cancer predisposition syndrome that increases the risk of multiple cancer types, including colorectal, prostate, uterine, and other malignancies.
DNA Damage Response Genes (CHEK2, ATM, PALB2, RAD51D)
This group of genes coordinates the cellular response to DNA damage, facilitating repair mechanisms that prevent malignant transformation. Mutations in these genes can compromise the cell’s ability to maintain genetic integrity, thereby increasing prostate cancer risk.
RNASEL Gene
The RNASEL gene regulates programmed cell death (apoptosis), ensuring that damaged cells are eliminated before they can cause harm. When inherited mutations disrupt this gene’s function, abnormal cells may escape destruction and potentially develop into prostate cancer.
HOXB13 Gene
This developmental gene influences prostate gland formation during embryogenesis. Rare inherited mutations in HOXB13 are associated with early-onset prostate cancer, typically occurring in men younger than 55 years of age.
Clinical Implications
Men with a family history of prostate, breast, or ovarian cancer may benefit from genetic counseling to assess their risk profile and determine appropriate screening strategies.¹⁸

The Benefits of Holistic Assessment
These tests and imaging tools are even more beneficial when combined with the Mederi Care Model of personalized constitutional assessment.
Throughout human history, indigenous medical systems across cultures have developed sophisticated diagnostic models based on energetic or non-tangible qualities.
These traditional frameworks include:
- Ayurveda: The three dosha system (Vata, Pitta, Kapha)
- Traditional Chinese Medicine (TCM): The five elements (earth, fire, metal, wood, and wind)
- European Humoral Medicine: The four temperaments (sanguine, melancholic, phlegmatic, choleric)
These time-tested approaches have contributed significantly to the development of Mederi Care, which synthesizes traditional wisdom with modern insights to create a comprehensive healing paradigm.
The Art of Constitutional Analysis
Constitutional assessment transcends rigid categorization. While established medical models offer valuable frameworks, the remarkable uniqueness of each individual necessitates a more flexible and integrative approach. Experienced holistic practitioners develop sophisticated diagnostic abilities that go beyond traditional classification systems, considering:
- Physical presentation and symptoms
- Emotional and psychological patterns
- Environmental influences
- Lifestyle factors
- Genetic predispositions
- Energetic qualities
The true artistry in holistic medicine lies in the practitioner’s ability to synthesize these diverse elements into a coherent understanding of the patient’s state of health. This holistic perspective extends beyond the immediate disease process to encompass the entirety of the patient’s well-being.
Plant Compounds May Reduce Unnecessary Prostate Biopsies
Elevated PSA levels often lead to prostate biopsies, many of which prove unnecessary. A study of 142 men with high PSA levels found that a combination of turmeric, boswellia, pine bark extract, and stinging nettle was more effective than saw palmetto alone:
- 76% of men taking the four-plant blend saw PSA levels drop (vs. 64% with saw palmetto)
- Only 35% of the plant combination group needed biopsies (vs. 78% with saw palmetto)
These results suggest specific plant compounds may help men with elevated PSA avoid unnecessary procedures while supporting prostate health naturally.¹⁹
Conclusion
The evolving understanding of prostate cancer challenges long-held beliefs about routine screening and aggressive treatment. Research suggests that a one-size-fits-all approach may not be the best strategy, as many prostate cancers grow slowly and may not pose a significant threat to a man’s health. While PSA testing remains a useful tool, it should be interpreted within a broader clinical context, incorporating additional diagnostic methods such as advanced panels, liquid biopsies, and MRIs.
As the field moves toward a more personalized approach, the focus is shifting from automatic cookie-cutter treatment to careful monitoring and targeted intervention. By considering factors such as cancer risk, quality of life, and emerging diagnostic technologies, patients and doctors can make more informed decisions. The future of prostate cancer care lies in a nuanced, individualized strategy that combines the best of conventional testing with the holistic constitutional assessments exemplified by Mederi Care.
About the Author:
Donald R. Yance is the founder of the Mederi Center. A Clinical Master Herbalist and Certified Nutritionist, Donnie is renowned for his extraordinary knowledge and deep understanding of the healing properties of plants and nutrition, as well as of epigenetics, laboratory medicine, oncologic pathology, and molecular oncology. He is a professional member of the American Herbalists Guild, National Association of Nutrition Professionals, Academy of Integrative Health and Medicine, and the Society for Integrative Oncology.
References:
- Ilic D, Djulbegovic M, Jung JH, Hwang EC, Zhou Q, Cleves A, Agoritsas T, Dahm P. Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018 Sep 5;362:k3519. doi: 10.1136/bmj.k3519. PMID: 30185521; PMCID: PMC6283370.
- Cooperberg MR. Re: Fifteen-year Outcomes After Monitoring, Surgery, or Radiotherapy for Prostate Cancer. European Urology. 2023;84(4):435-436. doi: 10.1016/j.eururo.2023.05.002
- Management of localised prostate cancer: watchful waiting, surgery or radiation therapy, depending on the natural course, which is often relatively slow. Prescrire Int. 2012 Oct;21(131):242-8. PMID: 23185849.
- Glass AS, Porten SP, Bonham M, Tran TC, Cowan JE, Punnen S, Chan JM, Carroll PR. Active surveillance: Does serial prostate biopsy increase histological inflammation? Prostate Cancer Prostatic Dis. 2013 Jun;16(2):165-169. doi: 10.1038/pcan.2012.51. Epub 2013 Jan 15.
- Ehdaie B, Vertosick E, Spaliviero M, Giallo-Uvino A, Taur Y, O’Sullivan M, Livingston J, Sogani P, Eastham J, Scardino P, Touijer K. The Impact of Repeat Biopsies on Infectious Complications in Men with Prostate Cancer on Active Surveillance. Journal of Urology. Sep 2013.
- Martin RM, Turner EL, Young GJ, Metcalfe C, Walsh EI, Lane JA, Sterne JAC, Noble S, Holding P, Ben-Shlomo Y, Williams NJ, Pashayan N, Bui MN, Albertsen PC, Seibert TM, Zietman AL, Oxley J, Adolfsson J, Mason MD, Davey Smith G, Neal DE, Hamdy FC, Donovan JL; CAP Trial Group. Prostate-Specific Antigen Screening and 15-Year Prostate Cancer Mortality: A Secondary Analysis of the CAP Randomized Clinical Trial. JAMA. 2024 May 7;331(17):1460-1470. doi: 10.1001/jama.2024.4011. PMID: 38581198
- Martin RM, Turner EL, Young GJ, et al. Prostate-Specific Antigen Screening and 15-Year Prostate Cancer Mortality: A Secondary Analysis of the CAP Randomized Clinical Trial. JAMA. 2024;331(17):1460–1470. doi:10.1001/jama.2024.4011
- Ventimiglia E, Gedeborg R, Styrke J, Robinson D, Stattin P, Garmo H. Natural history of nonmetastatic prostate cancer managed with watchful waiting. JAMA Netw Open. 2024;7(6). doi:10.1001/jamanetworkopen.2024.14599
- Ren ZJ, Cao DH, Zhang Q, Ren PW, Liu LR, Wei Q, et al. First-degree family history of breast cancer is associated with prostate cancer risk: a systematic review and meta-analysis. BMC Cancer. 2019;19(1):871. doi: 10.1186/s12885-019-6055-9
- Walz J, Haese A, Scattoni V, Steuber T, Chun FK, Briganti A, Montorsi F, Graefen M, Huland H, Karakiewicz PI. Percent free prostate-specific antigen (PSA) is an accurate predictor of prostate cancer risk in men with serum PSA 2.5 ng/mL and lower. Cancer. 2008 Nov 15;113(10):2695-703. doi: 10.1002/cncr.23885. PMID: 18853417.
- Belkahla S, Nahvi I, Biswas S, Nahvi I, Ben Amor N. Advances and development of prostate cancer, treatment, and strategies: A systematic review. Front Cell Dev Biol. 2022 Sep 9;10:991330. doi: 10.3389/fcell.2022.991330. PMID: 36158198; PMCID: PMC9501970.
- Strum S, Scholz M, McDermed J, et al. Modified citrus pectin slows PSA doubling time: A pilot clinical trial. Presentation: International conference on diet and Prevention of Cancer, Tampere, Finland. May 28, 1999-June 2, 1999.
- Roberts SG, Blute ML, Bergstralh EJ, Slezak JM, Zincke H. PSA doubling time as a predictor of clinical progression after biochemical failure following radical prostatectomy for prostate cancer. Mayo Clin Proc. 2001;76:576-581.
- Galletti G, Portella L, Tagawa ST, Kirby BJ, Giannakakou P, Nanus DM. Circulating tumor cells in prostate cancer diagnosis and monitoring: an appraisal of clinical potential. Mol Diagn Ther. 2014 Aug;18(4):389-402. doi: 10.1007/s40291-014-0101-8. PMID: 24809501; PMCID: PMC4149177.
- White J, et al. Clinical utility of the Prostate Health Index (phi) for biopsy decision management in a large group urology practice setting. Prostate Cancer and Prostatic Diseases. 2017.
- Robinson D, Abdulkareem R, Nasrollah D, et al. Frequency of Biopsy and Tumor Grade Before vs After Introduction of Prostate Magnetic Resonance Imaging. JAMA Netw Open. 2023;6(8):e2330233. doi:10.1001/jamanetworkopen.2023.30233
- Mayo Clinic. Prostate cancer diagnosis and treatment. Available at: https://www.mayoclinic.org/diseases-conditions/prostate-cancer/diagnosis-treatment/drc-20353093
- National Cancer Institute. Genetics of Prostate Cancer (PDQ®)–Health Professional Version. 2023. Available at: https://www.cancer.gov/types/prostate/hp/prostate-genetics-pdq
- Cai T, et al. Phytotherapy Might Have a Role in Reducing Unnecessary Prostate Biopsies. Clin Pract. 2024;14(1):188-197.
Additional References:
van Schooneveld E, Wildiers H, Vergote I, Vermeulen PB, Dirix LY, Van Laere SJ. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res. 2015 Feb 18;17:21. doi: 10.1186/s13058-015-0526-y. PMID: 25849621; PMCID: PMC4332424.
Kumar Am S, Rajan P, Alkhamees M, Holley M, Lakshmanan VK. Prostate cancer theragnostics biomarkers: An update. Investig Clin Urol. 2024 Nov;65(6):527-539. doi: 10.4111/icu.20240229. PMID: 39505512; PMCID: PMC11543649.
Höti N, Lih TS, Dong M, Zhang Z, Mangold L, Partin AW, et al. Urinary PSA and serum PSA for aggressive prostate cancer detection. Cancers (Basel). 2023;15:960. doi: 10.3390/cancers15030960.
Occhipinti S, Mengozzi G, Oderda M, Zitella A, Molinaro L, Novelli F, et al. Low levels of urinary PSA better identify prostate cancer patients. Cancers (Basel). 2021;13:3570. doi: 10.3390/cancers13143570.
Luo Y, Gou X, Huang P, Mou C. The PCA3 test for guiding repeat biopsy of prostate cancer and its cut-off score: a systematic review and meta-analysis. Asian J Androl. 2014 May-Jun;16(3):487-92. doi: 10.4103/1008-682X.125390. PMID: 24713827; PMCID: PMC4023384.
Wright JL, Chéry L, Holt S, Lin DW, Luedeke M, Rinckleb AE, Maier C, Stanford JL. Aspirin and NSAID use in association with molecular subtypes of prostate cancer defined by TMPRSS2:ERG fusion status. Prostate Cancer Prostatic Dis. 2016 Mar;19(1):53-6. doi: 10.1038/pcan.2015.49. Epub 2015 Oct 27. PMID: 26503111; PMCID: PMC4942258.
Perner S, et al. Am J Surg Pathol. 2007 Jun;31(6):882-8; Adv Anat Pathol. 2013 Mar;20(2):117-24.
Hendriks RJ, van Oort IM, Schalken JA. Blood-based and urinary prostate cancer biomarkers: a review and comparison of novel biomarkers for detection and treatment decisions. Prostate Cancer and Prostatic Diseases. 2017;20:12–19. doi:10.1038/pcan.2016.59