By Donnie Yance
Cancer becomes most dangerous when it spreads from its original location to other parts of the body—a process called metastasis. This spreading relies on certain proteins called lectins, which act like molecular “velcro” helping cancer cells stick together and communicate with each other. 1 Using modified citrus pectin for cancer is one strategy that can block or slow down the metastasis process.
One particular type of lectin—called galectin-3 (Gal-3)—has captured researchers’ attention because it plays a key role in how several types of cancers spread, including breast, prostate, and colon cancer.2, 3 Blood levels of galectin-3 closely track with cancer progression, making it a valuable marker for doctors to monitor how well treatments are working.
What is Modified Citrus Pectin?
Modified citrus pectin (MCP) is a natural substance extracted from the peel and pulp of citrus fruits like oranges, lemons, and grapefruits. Unlike regular pectin used in cooking, MCP is specially processed to break down into smaller molecules that can be better absorbed by the body.4
Scientists have discovered that MCP can block galectin-3, potentially interfering with cancer’s ability to spread.5 This blocking mechanism is like removing the “velcro” that cancer cells use to stick together and attach to other tissues in the body.
MCP produces multiple effects, including but not limited to its antagonism of galectin-3, which have shown benefit in preclinical and clinical models. Regarding cancer, MCP modulates several rate-limiting steps of the metastatic cascade. MCP can also affect cancer cell resistance to chemotherapy.6
How MCP Works Against Cancer
When cancer cells break away from their original tumor, they travel through the bloodstream until becoming trapped in small blood vessels. There, they use galectin-3 to attach to the blood vessel walls, penetrate through them, and establish new tumor colonies.7,8
MCP appears to interrupt this process by binding to galectin-3 on cancer cells, preventing them from attaching to blood vessel walls and blocking their ability to form new tumor colonies.9 This interference happens without harming normal cells.
Research on MCP and Different Cancers
Prostate Cancer
Several studies show promising results for MCP in prostate cancer:
- In laboratory studies, MCP prevented prostate cancer cells from attaching to other cells, a critical step in metastasis11
- In animal studies, rats given MCP had significantly fewer lung metastases compared to untreated animals12
- In a human study, 70% of men with rising PSA levels (a prostate cancer marker) showed significant slowing in their PSA doubling time after taking MCP for 12 months13
- An 18-month clinical trial found that 85% of participants with early-stage prostate cancer showed positive results with MCP treatment, including slower cancer progression markers and no visible spread14
Researchers conducted a study on men with early-stage prostate cancer who had rising PSA levels (a blood marker for prostate cancer) after initial treatment but no visible spread of cancer. This condition, while not immediately life-threatening, often leaves patients and doctors uncertain about the best treatment approach.
In this study:
- Patients took PectaSol® (a specific brand of modified citrus pectin) three times daily for 18 months
- After the full treatment period, 85% of patients showed positive results
- 62% had PSA levels that either decreased or remained stable
- 90% showed improvement in how quickly their PSA levels were rising (slower increases are better)
- All patients remained free of visible cancer spread
- No serious side effects were reported.15
Breast Cancer
Research shows that MCP may help prevent breast cancer cells from spreading:
- Laboratory studies demonstrate that MCP blocks breast cancer cells from adhering to blood vessel walls16,17
- Higher grades of invasive breast cancer show increased galectin-3 expression, suggesting MCP could be particularly beneficial18
- A systematic review of studies over 22 years found positive results for MCP against breast cancer in both laboratory and animal studies.19
Other Cancers
MCP has also shown promise with:
- Melanoma: In one animal study, MCP decreased tumor spread to the lungs by more than 90% 20
- Colon Cancer: MCP effectively inhibited liver metastasis in laboratory models21
- Ovarian Cancer: Studies show MCP increases the effectiveness of standard chemotherapy drugs.22
- Bladder cancer: MCP inhibited bladder cancer tumor development by causing cell cycle arrest and death in vitro and in vivo. In high-quality bladder cancer samples, MCP reduced the expression of Gal-3, which has functions related to cell proliferation, survival, adhesion, and metastasis.23,24
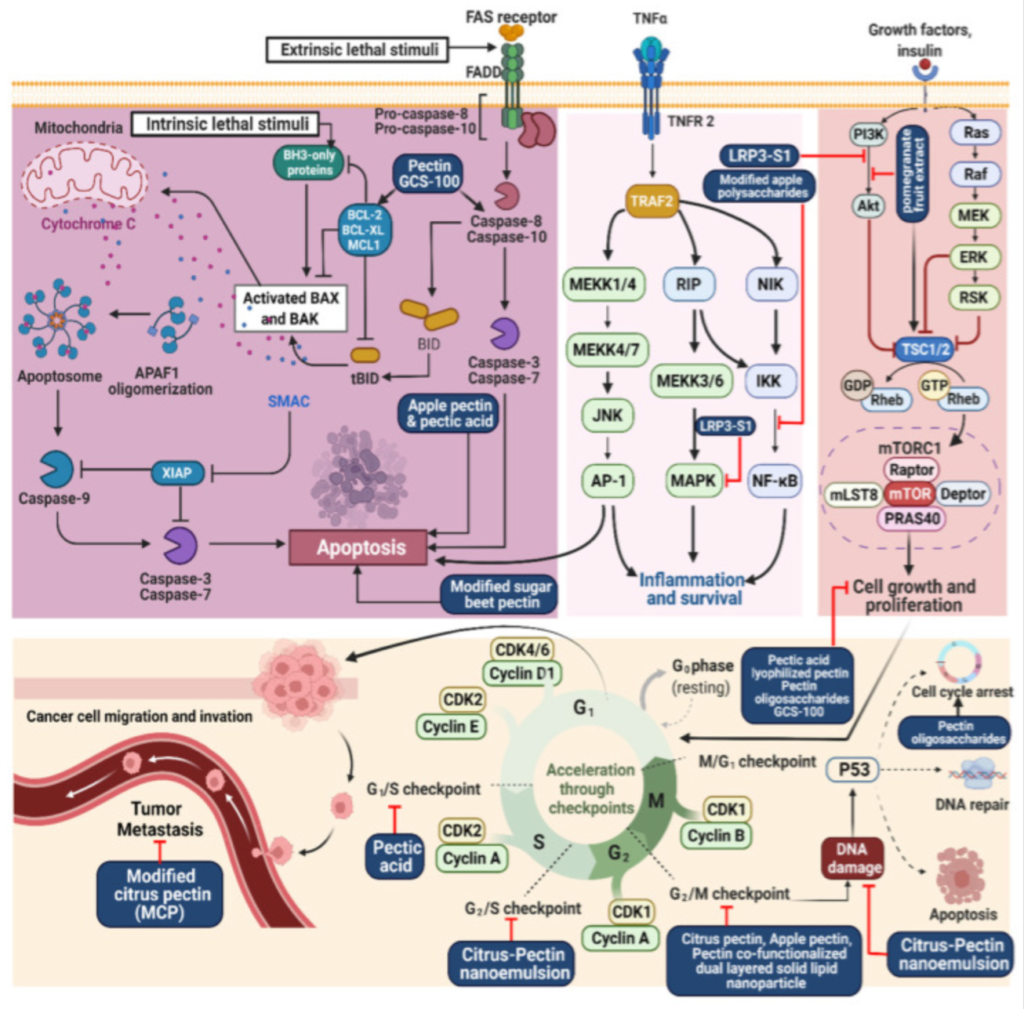
Illustration representing the site of action of modified citrus pectin and associated pectins in cancer 25 treatment.
Beyond Cancer: Other Health Benefits
- Kidney Health: Research shows MCP reduces kidney injury and protects against damage from diabetes26,27
- Myocardial fibrosis (MF) plays a key role in the development and progression of heart failure (HF) with limited effective therapies. Galectin-3 (Gal-3) is a biomarker associated with fibrosis and inflammation in patients with HF. MCP protects against cardiac dysfunction, through downregulating Gal-3 expression and suppressing activation of pro-inflammatory signaling pathway28
- Anti-inflammatory Effects: MCP appears to reduce harmful inflammation throughout the body 29
- Potential for Combination Therapy: MCP may enhance conventional cancer treatments with minimal side effects.30,31,32
- Heavy Metal Detoxification: MCP binds to and enhances excretion of toxic metals like lead, mercury, arsenic, and cadmium without depleting essential minerals.33, 34
- Immunomodulation: Activates T-cells and NK cells involved in anti-cancer immunity. 35
- Gastrointestinal Health: Supports healthy gut bacteria by providing fermentable Prebiotic fiber. 36
- Cell adhesion inhibition: Can prevent pathogen cell adhesion to tissues. MCP was shown to reduce the adhesiveness of toxic E coli to the intestinal wall. 37
- May Help Prevent and Treat Atherosclerosis: In mouse models, MCP reduced the size of atherosclerotic lesions by inhibiting the adhesion of white blood cells to the cells inside of blood vessel walls. 38
- Potential Neuroprotective Effects (early research): Galectin-3 has been implicated in neurodegeneration, and MCP may help by reducing neuroinflammation. 39
Safety and Side Effects
MCP is generally well-tolerated, even at high doses. Since it’s a soluble fiber, the most common side effect is occasional loose stools, which typically resolves without stopping treatment. The FDA categorizes MCP as “Generally Recognized as Safe” (GRAS).40,41
Recommended Dosage
In clinical studies showing benefits for prostate cancer, doses of approximately 15 grams per day were used.42,43 The powder is usually dissolved in water or juice.
Clinical studies on MCP have generally used doses ranging from 5-15 grams per day, typically divided into 2-3 doses:
- Standard preventive dose: 5 grams daily (approximately 1-2 teaspoons), divided into morning and evening doses
- Therapeutic dose for active cancer: 15 grams daily (approximately 1-2 tablespoons), divided into three doses
- Treatment duration: Most clinical trials have administered MCP for periods of 3-18 months with continued monitoring
For optimal absorption, MCP should be taken on an empty stomach (30 minutes before meals or 2 hours after) mixed in water or juice. Effects on cancer markers like PSA doubling time may take 3-6 months to become apparent, suggesting the importance of consistent, long-term use.
These results are encouraging because they suggest that modified citrus pectin, a natural food supplement considered safe by the FDA, might offer a gentle yet effective option for inhibiting or slowing down cancer growth without significant toxicity.44
Further Reading
If you would like to take a deeper dive into learning more about Modified Citrus Pectin and Galectin-3 and their health effects, Dr Isaac Eliaz has written a book called The Survival Paradox: Reversing the Hidden Cause of Aging and Chronic Disease, Dr Eliaz reveals how the body’s innate survival response, while essential for immediate protection, can, when chronically activated, lead to inflammation, disease progression, and premature aging. Drawing on decades of clinical experience and scientific research, he introduces the concept of Galectin-3 as a key biomarker and driver of this survival response. Through practical tools, including detoxification, targeted nutrition, stress reduction, and the use of Modified Citrus Pectin, Dr Eliaz teaches how to calm the body’s survival mode and activate its natural healing potential.
Conclusion
Modified citrus pectin (MCP) offers a promising and natural way to help fight the spread of cancer and other conditions. By blocking galectin-3, MCP can interfere with how cancer cells grow, stick to each other, and travel through the body. Research shows that MCP may slow down or even stop cancer progression in several types of cancer, including prostate, breast, and colon cancers. It also appears to support kidney, heart, and immune system health, while helping the body safely remove toxic heavy metals. Best of all, MCP is well-tolerated, safe for most people, and can be used alongside other cancer treatments. As science continues to explore the benefits of MCP, it’s on its way to becoming an important part of a well-rounded plan to reverse chronic disease.
About the Author:
Donald R. Yance is the founder of the Mederi Center. A Clinical Master Herbalist and Certified Nutritionist, Donnie is renowned for his extraordinary knowledge and deep understanding of the healing properties of plants and nutrition, as well as of epigenetics, laboratory medicine, oncologic pathology, and molecular oncology. He is a professional member of the American Herbalists Guild, National Association of Nutrition Professionals, Academy of Integrative Health and Medicine, and the Society for Integrative Oncology.
References:
1. Raz a, Loton R. Endogenous galactoside-binding lectins: a new class of functional cell surface molecules related to metastasis. Cancer Metastasis Rev 1987;6:433-452.
2. Idikio H. Galectin-3 expression in human breast carcinoma: correlation with cancer histologic grade. Int J Oncol 1998;12:1287-1290.
3. Nangia-Makker P, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol 2000;156:899–909.
4. Nicolson GL. Cancer metastasis: tumor cell and host organ properties important in metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J Natl cancer Inst 1995;87:348-353.
5. Gao X, Zhi Y, Zhang T, Xue H, Wang X, Foday AD, Tai G, Zhou Y. Analysis of the neutral polysaccharide fraction of MCP and its inhibitory activity on galectin-3. Glycoconj J. 2012 May;29(4):159-65.
6. Eliaz I, Raz A. Pleiotropic Effects of Modified Citrus Pectin. Nutrients. 2019 Nov 1;11(11):2619. doi: 10.3390/nu11112619. PMID: 31683865; PMCID: PMC6893732.
7. Raz a, Loton R. Endogenous galactoside-binding lectins: a new class of functional cell surface molecules related to metastasis. Cancer Metastasis Rev 1987;6:433-452.
8. Nicolson GL. Cancer metastasis: tumor cell and host organ properties important in metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J Natl cancer Inst 1995;87:348-353.
9. Naik H, Pilat MF, donat T, et al. Inhibition of in vitro tumor cell-endothelial adhesion by modified citrus pectin: a pH modified natural complex carbohydrate. Proc Am Assoc Cancer Res 1995:36:Abstract 377.
10. Glinsky VV, Raz A. Modified citrus pectin anti-metastatic properties: one bullet, multiple targets. Carbohydr Res. 2009 Sep 28;344(14):1788-91. doi: 10.1016/j.carres.2008.08.038. Epub 2008 Sep 26. PMID: 19061992; PMCID: PMC2782490.
11. Pienta KF, Naik H, Akhtah A, et al. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J Natl Cancer Inst 1995;87:348-353.
12. Pienta KF, Naik H, Akhtah A, et al. Inhibition of spontaneous metastasis in a rat prostate cancer model by oral administration of modified citrus pectin. J Natl Cancer Inst 1995;87:348-353
13. Brad Guess, Mark Scholz, Richard Lam and Henry Johnson. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: a phase II pilot study. Prostate Cancer Prostatic Dis. 2003;6(4):301-4.
14. Keizman D, Frenkel M, Peer A, et al. Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Long-Term Results of a Prospective Phase II Study. Nutrients. 2023 Aug 11;15(16):3533.
15. Keizman D, Frenkel M, Peer A, Rosenbaum E, Sarid D, Leibovitch I, Mano R, Yossepowitch O, Wolf I, Geva R, Margel D, Rouvinov K, Stern A, Dresler H, Kushnir I, Eliaz I. Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Long-Term Results of a Prospective Phase II Study. Nutrients. 2023 Aug 11;15(16):3533. doi: 10.3390/nu15163533. PMID: 37630724; PMCID: PMC10459199.
16. Naik H, Pilat MF, donat T, et al. Inhibition of in vitro tumor cell-endothelial adhesion by modified citrus pectin: a pH modified natural complex carbohydrate. Proc Am Assoc Cancer Res 1995:36:Abstract 377.
17. Glinsky VV, Huflejt ME, Glinsky GV, et al. Effects of Thomsen-Friedenreich antigen-specific peptide P-30 on beta-galatoside-mediated homotypic aggregation and adhesion to the endothelium of MDA-MB-435 human breast carcinoma cells. Cancer Res 2000;60:2584-2588.
18. Idikio H. Galectin-3 expression in human breast carcinoma: correlation with cancer histologic grade. Int J Oncol 1998;12:1287-1290.
19. Garrido G, Garrido-Suárez BB, Mieres-Arancibia M, et al. Modified pectin with anticancer activity in breast cancer: A systematic review. Int J Biol Macromol. 2024 Jan;254(Pt 1):127692.
20. Platt D, Raz A. Modulation of the lung cell colonization of B16-F1 melanoma cells by citrus pectin. J Natl Cancer Inst 1992;18:438-442.
21. Nangia-Makker P, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol 2000;156:899–909.
22. Hossein G, Keshavarz M, Ahmadi S, Naderi N. Synergistic Effects of PectaSol-C Modified Citrus Pectin an Inhibitor of Galectin-3 and Paclitaxel on Apoptosis of Human SKOV-3 Ovarian Cancer Cells. Asian Pac J Cancer Prev. 2013;14(12):7561-8.
23. Cardoso A.C.F., Andrade L.N.d.S., Bustos S.O., Chammas R. Galectin-3 determines tumor cell adaptive strategies in stressed tumor microenvironments. Front. Oncol. 2016;6:6. doi: 10.3389/fonc.2016.00127.
24. Wang L., Guo X.L. Molecular regulation of galectin-expression and therapeutic implication in cancer progression. Biomed. Pharmacother. 2016;78:165–171. doi: 10.1016/j.biopha.2016.01.014
25. Emran TB, Islam F, Mitra S, Paul S, Nath N, Khan Z, Das R, Chandran D, Sharma R, Lima CMG, Awadh AAA, Almazni IA, Alhasaniah AH, Guiné RPF. Pectin: A Bioactive Food Polysaccharide with Cancer Preventive Potential. Molecules. 2022 Oct 31;27(21):7405. doi: 10.3390/molecules27217405. PMID: 36364232; PMCID: PMC9657392.
26. Mahmoud HM, Abdel-Razik AH, Elrehany MA, et al. Modified Citrus Pectin (MCP) Confers a Renoprotective Effect on Early-Stage Nephropathy in Type-2 Diabetic Mice. Chem Biodivers. 2024 Jul;21(7):e202400104.
27. Kolatsi-Joannou M, Price KL, Winyard PJ, Long DA (2011) Modified Citrus Pectin Reduces Galectin-3 Expression and Disease Severity in Experimental Acute Kidney Injury. PLoS ONE 6(4): e18683.
28. Xu GR, Zhang C, Yang HX, Sun JH, Zhang Y, Yao TT, Li Y, Ruan L, An R, Li AY. Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-κB signaling pathway. Biomed Pharmacother. 2020 Jun;126:110071. doi: 10.1016/j.biopha.2020.110071. Epub 2020 Mar 12. PMID: 32172066.
29. Kolatsi-Joannou M, Price KL, Winyard PJ, Long DA (2011) Modified Citrus Pectin Reduces Galectin-3 Expression and Disease Severity in Experimental Acute Kidney Injury. PLoS ONE 6(4): e18683.
30. Leclere L, Cutsem PV, Michiels C. Anti-cancer activities of pH- or heat-modified pectin. Front Pharmacol. 2013 Oct 8;4:128.
31. Yan J, Katz A. PectaSol-C modified citrus pectin induces apoptosis and inhibition of proliferation in human and mouse androgen-dependent and-independent prostate cancer cells. Integr Cancer Ther. 2010;9:197–203.
32. Glinsky VV, Raz A. Modified citrus pectin anti-metastatic properties: one bullet, multiple targets. Carbohydr Res. 2009 Sep 28;344(14):1788-91. doi: 10.1016/j.carres.2008.08.038. Epub 2008 Sep 26. PMID: 19061992; PMCID: PMC2782490.
33. Zhao ZY, Liang L, Fan X, Yu Z, Hotchkiss AT, Wilk BJ, Eliaz I. The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Altern Ther Health Med. 2008 Jul-Aug;14(4):34-8. Erratum in: Altern Ther Health Med. 2008 Nov-Dec;14(6):18. PMID: 18616067.
34. Eliaz I, Hotchkiss AT, Fishman ML, Rode D. The effect of modified citrus pectin on urinary excretion of toxic elements. Phytother Res. 2006 Oct;20(10):859-64. doi: 10.1002/ptr.1953. PMID: 16835878.
35. Ramachandran C, Wilk BJ, Hotchkiss A, Chau H, Eliaz I, Melnick SJ. Activation of human T-helper/inducer cell, T-cytotoxic cell, B-cell, and natural killer (NK)-cells and induction of natural killer cell activity against K562 chronic myeloid leukemia cells with modified citrus pectin. BMC Complement Altern Med. 2011 Aug 4;11:59. doi: 10.1186/1472-6882-11-59. PMID: 21816083; PMCID: PMC3161912.
36. Ho YY, Lin CM, Wu MC. Evaluation of the prebiotic effects of citrus pectin hydrolysate. J Food Drug Anal. 2017 Jul;25(3):550-558. doi: 10.1016/j.jfda.2016.11.014. Epub 2017 Feb 13. PMID: 28911641; PMCID: PMC9328821.
37. Di R, Vakkalanka MS, Onumpai C, Chau HK, White A, Rastall RA, Yam K, Hotchkiss AT Jr. Pectic oligosaccharide structure-function relationships: Prebiotics, inhibitors of Escherichia coli O157:H7 adhesion and reduction of Shiga toxin cytotoxicity in HT29 cells. Food Chem. 2017 Jul 15;227:245-254. doi: 10.1016/j.foodchem.2017.01.100. Epub 2017 Jan 21. PMID: 28274429.
38. Lu Y, Zhang M, Zhao P, Jia M, Liu B, Jia Q, Guo J, Dou L, Li J. Modified citrus pectin inhibits galectin-3 function to reduce atherosclerotic lesions in apoE-deficient mice. Mol Med Rep. 2017 Jul;16(1):647-653. doi: 10.3892/mmr.2017.6646. Epub 2017 May 29. PMID: 28560429; PMCID: PMC5482107.
39. Lozinski BM, Ta K, Dong Y. Emerging role of galectin 3 in neuroinflammation and neurodegeneration. Neural Regen Res. 2024 Sep 1;19(9):2004-2009. doi: 10.4103/1673-5374.391181. Epub 2023 Dec 21. PMID: 38227529; PMCID: PMC11040290.
40. Strum S, Scholz M, McDermed J, et al. Modified citrus pectin slows PSA doubling time: A pilot clinical trial. Presentation: International conference on diet and Prevention of Cancer, Tampere, Finland. May 28, 1999-June 2, 1999.
41. Keizman D, Frenkel M, Peer A, et al. Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Long-Term Results of a Prospective Phase II Study. Nutrients. 2023 Aug 11;15(16):3533.
42. Strum S, Scholz M, McDermed J, et al. Modified citrus pectin slows PSA doubling time: A pilot clinical trial. Presentation: International conference on diet and Prevention of Cancer, Tampere, Finland. May 28, 1999-June 2, 1999.
43. Brad Guess, Mark Scholz, Richard Lam and Henry Johnson. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: a phase II pilot study. Prostate Cancer Prostatic Dis. 2003;6(4):301-4.
44. Keizman D, Frenkel M, Peer A, Rosenbaum E, Sarid D, Leibovitch I, Mano R, Yossepowitch O, Wolf I, Geva R, Margel D, Rouvinov K, Stern A, Dresler H, Kushnir I, Eliaz I. Modified Citrus Pectin Treatment in Non-Metastatic Biochemically Relapsed Prostate Cancer: Long-Term Results of a Prospective Phase II Study. Nutrients. 2023 Aug 11;15(16):3533. doi: 10.3390/nu15163533. PMID: 37630724; PMCID: PMC10459199.

