Fifty percent of all drugs, including cancer drugs marketed today, use the cytochrome P450 enzyme pathway for metabolism[1],[2] and frequently cause interactions. In brief, the cytochrome P4503A (CYP3A) subfamily is largely found in hepatocytes, with some presence in the intestine. Together with the transport protein P-glycoprotein (PGP) present in the small intestine, these 2 systems regulate metabolism of drugs and nutrients. Many foods, food components, and botanical supplements interact with the CYP3A and PGP metabolism, but even more importantly, an individual’s genome plays a significant role in how well one metabolizes and detoxifies various drugs including Aromatase and mTOR inhibitors. The effects of herbs on CYP3A and PGP is dose sensitive, meaning that often the dose would need to be very high in order to really impact these pathways. Another important factor is that much of the published research is either in vitro; animal in vivo, done with isolates (not whole herbs), and/or based on high dosages of these compounds. So, every referenced paper needs to be reviewed and analyzed for accuracy and relativity to how botanicals might impact both the effectiveness or toxicity of these and other drugs. The other factor is that to base interaction solely on P4503A and PGP is wrong. The ultimate question is still not answered, which is does this herbal combination either cause this drug to be less effective or does it increase the toxicity? In general, due to these nuances, evaluating the potential for negative drug-drug, nutrient-drug, or botanical-drug interactions metabolized through this pathway can be difficult and often lead to incorrect assumptions.
A previous blog of mine entitled, “The Truth about Herb-Drug Interactions: An Honest Evaluation of the Benefits and Risks When Weighing Scientific Data and Clinical Experience” https://www.donnieyance.com/the-truth-about-herb-drug-interactions-an-honest-evaluation-of-the-benefits-and-risks-when-weighing-scientific-data-and-clinical-experience/ provides critical backround for understanding the “good news” regarding the benefits of herb-and-drug combinations, and specifically curcumin rich turmeric extract and chemotherapy.
Many chemotherapeutic drugs have been used for the treatment of cancer, including doxorubicin, irinotecan, 5-fluorouracil, cisplatin, and paclitaxel. However, the effectiveness of chemotherapy is limited in cancer therapy due to drug resistance, therapeutic selectivity, and undesirable side effects.
Chemosensitization is one valuable strategy to overcome chemoresistance phenomena. Chemosensitization is based on the use of one drug to enhance the activity of another by influencing one or more mechanisms of resistance. The combination of therapies with natural compounds such as curcumin is likely to increase the effectiveness of drug treatment as well as reduce the incidence of adverse outcomes.
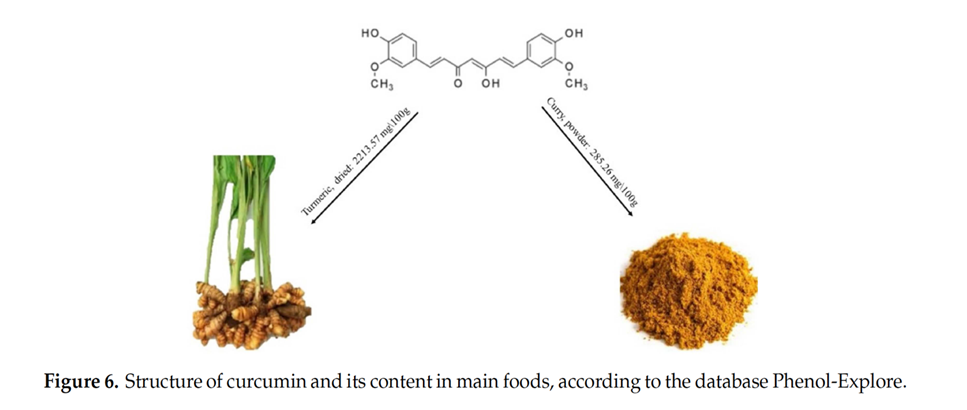
Curcumin, a polyphenolic isolated from Curcuma longa, belongs to the rhizome of Zingiberaceae plants.
Both in vitro and in vivo studies show that curcumin exerts many pharmacological activities with less toxic effects. Chemosensitization has been observed in cancers of the breast, colon, pancreas, gastric, liver, blood, lung, prostate, bladder, cervix, ovary, head and neck, and brain and in multiple myeloma, leukemia, and lymphoma. Similar studies have also shown that curcumin can sensitize a variety of tumors to gamma radiation including glioma, neuroblastoma, cervical carcinoma, epidermal carcinoma, prostate cancer, and colon cancer. The way in which curcumin acts as a chemosensitizer and radiosensitizer has been studied extensively. For example, it downregulates various growth regulatory pathways and specific genetic targets including genes for NF-κB, STAT3, COX2, Akt, antiapoptotic proteins, growth factor receptors, and multidrug-resistance proteins. Although it acts as a chemosensitizer and radiosensitizer for tumors in some cases, curcumin has also been shown to protect healthy organs such as the liver, kidney, oral mucosa, and heart from chemotherapy and radiotherapy-induced toxicity.[3]
The Bioavailability of Curcumin: Separating Truth from Fiction
Curcumin and other curcuminoids from Curcuma longa (turmeric) are important bioactive compounds exhibiting various pharmacological activities. Turmeric is widely consumed as part of the spice mix curry and as a dietary supplement. It has a long history of therapeutic application in traditional Asian medicine. The active components of turmeric, collectively known as curcuminoids, are among the most promising natural compounds studied across the globe today.
Clinical studies support the active role of curcuminoids in the management of chronic health conditions. Several pioneering studies have been conducted by major universities, research institutes and hospitals on Curcumin C3 Complex®, which have aided the understanding of the metabolic effects of curcumin.
Curcumin is a natural product with multiple biological activities and numerous potential therapeutic applications. However, the poor systemic bioavailability of the product fails to explain the potent pharmacological effects and hinders its clinical application.
The biological effects of curcumin in cellular and animal models are surprising considering its chemical and metabolic instability. Multiple studies have shown that, even with high doses of curcumin, the levels of curcumin in serum and tissues as well as its in vivo metabolites are extremely low after a short period of time.
With a lack of consensus on curcumin bioavailability and with various formulations making a variety of claims on the bioavailability (X times or XX times more than nearest competitor), consumers are understandably confused.
The reality is that after ingestion, little if any curcumin is present unchanged in systemic circulation.Furthermore, curcumin undergoes rapid non-enzymatic degradation in cell culture medium and possibly in vivo as well. Chemical transformation by human gut microbiota does not mean a loss in activity, but is actually a necessity for the therapeutic action of curcumin. Current thinking is that the molecular mechanisms of degradation of curcumin is necessary for interpreting in vitro and in vivo studies.
Compared to the parent compound curcumin, the degradation products mixture possessed higher O2.–-scavenging activity and stronger inhibition against fAβ formation. The docking simulations revealed that the bioactive degradation products make an important contribution to the experimentally observed enzymatic inhibition activities of curcumin.
Curcumin has been shown to possess low stability in aqueous solutions at physiological pH and degrades readily.. In phosphate buffer at pH 7.4, about 90% of curcumin degraded within 30 min and the degradation products have been identified as trans-6-(4′-hydroxy-3′-methoxyphenyl)-2,4-dioxo-5-hexenal, ferulic aldehyde, ferulic acid, feruloyl methane, vanillin, vanillic acid, and other dimerization end-products.
Selected degradation products mentioned above were the major human metabolites after curcumin consumption, and their levels were much higher than those of its metabolic compounds.
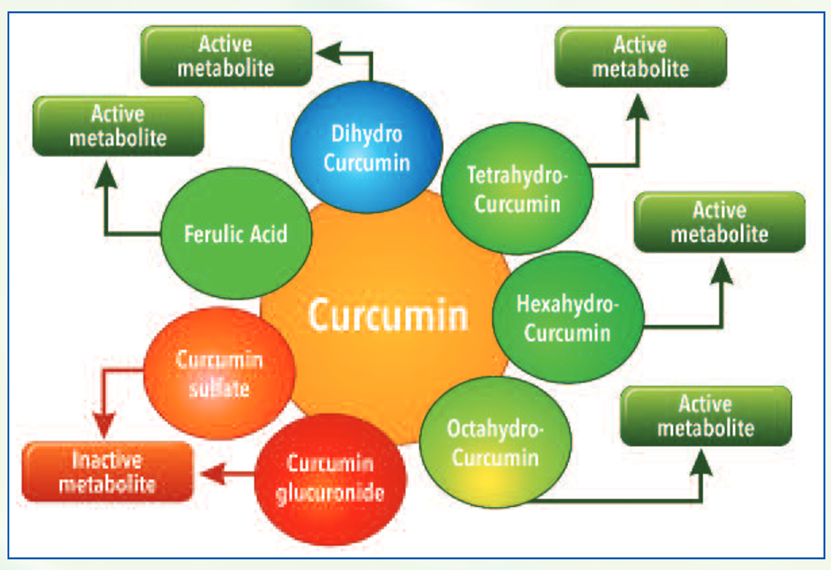
Upon ingestion, curcumin forms several active metabolites undergoing phase I metabolism such as dihydrocurcumin, tetrahydrocurcumin, hexahydrocurcumin and octahydrocurcumin.
In course of its metabolism, it also forms degradation compounds such as ferulic acid and bicyclopentadione. While these phase I metabolites have shown to have beneficial biological activity, compounds such as curcumin glucuronides and sulfates formed after phase II metabolism have shown to be ineffective in independent studies.
Given that curcumin is readily degraded under physiological condition, recent findings strongly suggested that the degradation products should make important contribution to the diverse biological activities of curcumin.[4],[5],[6],[7]
Oral curcumin also improved cachexia and general health in colorectal cancer patients.[8] In a phase II trial involving 21 patients with advanced pancreatic cancer, curcumin demonstrated bioactivity by downregulating nuclear factor-κB and cyclooxygenase-2. Despite limited absorption, antitumor response was seen in two patients.[9]
Curcumin Combined with Various Chemotherapeutic Agents
Combined with 5-FU
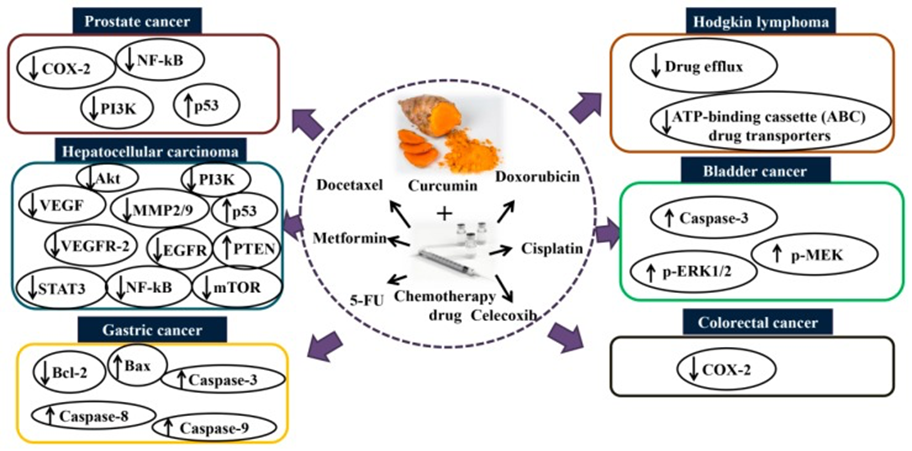
- Research suggests that the combination of 5-FU and curcumin may overcome drug-resistance induced by 5-FU. Pre-treatment with curcumin (5 µM) enhanced the chemosensitization of 5-FU (0.1 µM) and reversed the multidrug resistance in resistant mismatch repair (MMR)-deficient human colon cancer cells compared to 5-FU alone.[10]
- The combination of curcumin (10 μM) and 5-FU (0.1 mM)/oxaliplatin (5 μM) enhanced the synergistic antitumor activity in gastric cancer (BGC-823) cell lines compared to curcumin or 5-FU/oxaliplatin alone by downregulating the Bcl-2 mRNA and protein expression and activating the Bax and caspases-3, 8, and 9 expressions. This study further demonstrated that the combination of curcumin (10 mg/kg) and 5-FU (33 mg/kg)/oxaliplatin (10 mg/kg) shows potent growth inhibition of BGC-823 xenograft tumors compared to folinic acid, 5-FU, oxaliplatin (FOLFOX) or curcumin alone. [11]
- A combination of curcumin (50 mg/kg/day for 40 days) and 5-FU (20 mg/kg once every 2 days for 40 days) inhibits cell proliferation against 5-FU resistant cells via suppression of epithelial-to-mesenchymal transition (EMT) compared to 5FU alone.[12]
- In the context of breast cancer, a combination of curcumin (10 µM) and 5-FU (10 µM) significantly inhibited cell viability and enhanced apoptosis compared to 5-FU alone in vitro.[13]
- In addition to the effects mentioned above, research indicates that combining 5-FU (50 µmol/L) and curcumin (25 µmol/L) can enhance the cytotoxicity against human gastric cancer (AGS) cells compared to 5-FU or curcumin alone.
- In a further study focused on inflammation outcomes, this study found that the protein expression of COX-2 and NF-κB in human gastric cancer (MKN45) cells were diminished after co-treatment with 5-FU (50 µmol/L) and curcumin (25 µmol/L). This finding implies that curcumin sensitizes gastric cancer cells to 5-FU by modulating inflammatory molecules. The anti-gastric cancer activity is shown not only in in vitro study, but data from an animal study further demonstrated that curcumin enhanced the anticancer activity of 5-FU (52 mg/kg 5-FU + curcumin 74 mg/kg, every 3 days for 6 times in total) compared to 5-FU or curcumin alone, and without increasing the toxicity in nude mice bearing MKN45 tumor xenografts.[14]
Combined with Doxorubicin
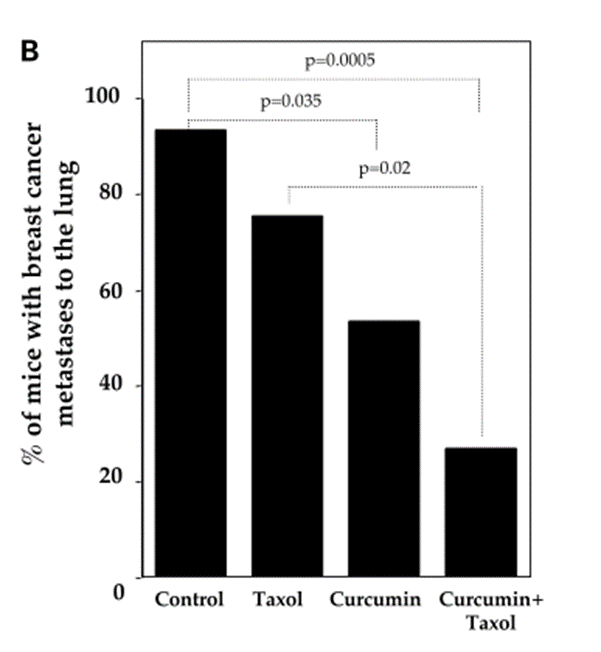
Doxorubicin, one of the active single-agent drugs, is widely used for the treatment of cancers, including leukemia, lung, brain, prostate, ovarian, and breast. However, the clinical use of doxorubicin often led to critical cardiotoxicity and developed multidrug resistance. Substantial evidence showed that curcumin (4 mg/kg every 2 days for a total of 7 injections) exhibits a better treatment efficacy of doxorubicin (0.4 mg/kg) in cancer due to the efflux inhibitory effect of curcumin.[15],[16],[17],[18]
A study conducted by Guorgui et al.[19] has shown that combination of curcumin (5 µM) and doxorubicin (0.4 mg/mL) demonstrated a stronger additive effect by reducing the proliferation of Hodgkin lymphoma (L-540) cells by 79%. The pharmacokinetic study also revealed that curcumin (5 mg/kg) could enhance the absorption of doxorubicin (5 mg/kg) and decrease drug efflux in vivo, suggesting that curcumin downregulates the intracellular levels of ATP-binding cassette (ABC) drug transporters.[20]
While DOX alone does not decrease tumor weight, the combination of DOX and curcumin has been shown to significantly reduce tumor weight to 56.5% (p<0.05) to that of the control group. In combination, curcumin enhanced apoptosis by DOX and decreased cell viability. The curcumin-DOX combination also suppressed activation of caspase-3, -8, and -9 compared to DOX alone. It appears that curcumin increases DOX-induced antitumor activity by suppressing the main caspase pathway and activating the main caspase independent pathway. The combination of curcumin and DOX suppressed the reduction of glutathione peroxidase activity and increased lipid peroxide levels in the heart. Therefore, it is expected that curcumin may reduce the adverse reactions associated with DOX. According to researchers, results suggest that curcumin can be used as a modulator to enhance the therapeutic index of cancer patients and improve their QOL.[21]
Combined with Platin Agents
Cisplatin-based combination therapy has emerged as a standard therapy for metastatic and advanced bladder cancer,[22] demonstrating 15–20% improved survival and 50–70% response rate. However, nearly 30% of patients do not respond to initial chemotherapy and show recurrence within 1 year.[23] Cisplatin is an inorganic platinum agent which can induce DNA-protein and interstrand and intrastrand DNA crosslinks.[24]
While this crosslink can induce apoptosis and inhibit cell proliferation,[25] the efficacy of cisplatin is limited by the development of cell resistance. Co-treatment with curcumin (10 µM) and cisplatin (10 µM) has shown a potent synergistic effect by activating caspase-3 and upregulating phospho-mitogen-activated protein kinase (p-MEK) and phospho-extracellular signal-regulated kinase 1/2 (p-ERK1/2) signaling in bladder cancer cell lines (253J-Bv and T24) compared to curcumin or cisplatin alone.[26] In addition to the effects observed on bladder cancer, the combination of curcumin and cisplatin was shown to upregulate the expression of miR-186 via modulation of Twist1 in ovarian cancer compared to cisplatin alone.[27]
Synergism of Curcumin and Epigallocatechin-3-gallate
Drug resistance remains an ongoing challenge in ovarian cancer chemotherapy. Research has investigated the synergism in activity from the sequenced combinations of cisplatin (Cis) with curcumin (Cur) and epigallocatechin-3-gallate (EGCG) in ovarian cancer cell lines. The drugs were added in binary combinations: Cis combined with Cur, and Cis combined with EGCG to the human ovarian A2780 and A2780(cisR) cancer cell lines, using five different sequences of administration: 0/0 h, 4/0 h, 0/4 h, 24/0 h and 0/24 h. Addition of Cis 4 h before Cur and EGCG (0/4 h combination) produced the most synergistic outcomes in both the A2780 and A2780(cisR) cell lines. The cellular accumulations of platinum and platinum-DNA binding resulting from the 0/4 h combinations were greater as compared to the values using Cis alone, thus providing an explanation for the synergistic action. When sequenced combinations of Cis with Cur and with EGCG are applied to human ovarian A2780 and A2780(cisR) cancer cell lines, lower concentrations and shorter time gap between the two additions seem to produce a higher cytotoxic effect.[28]
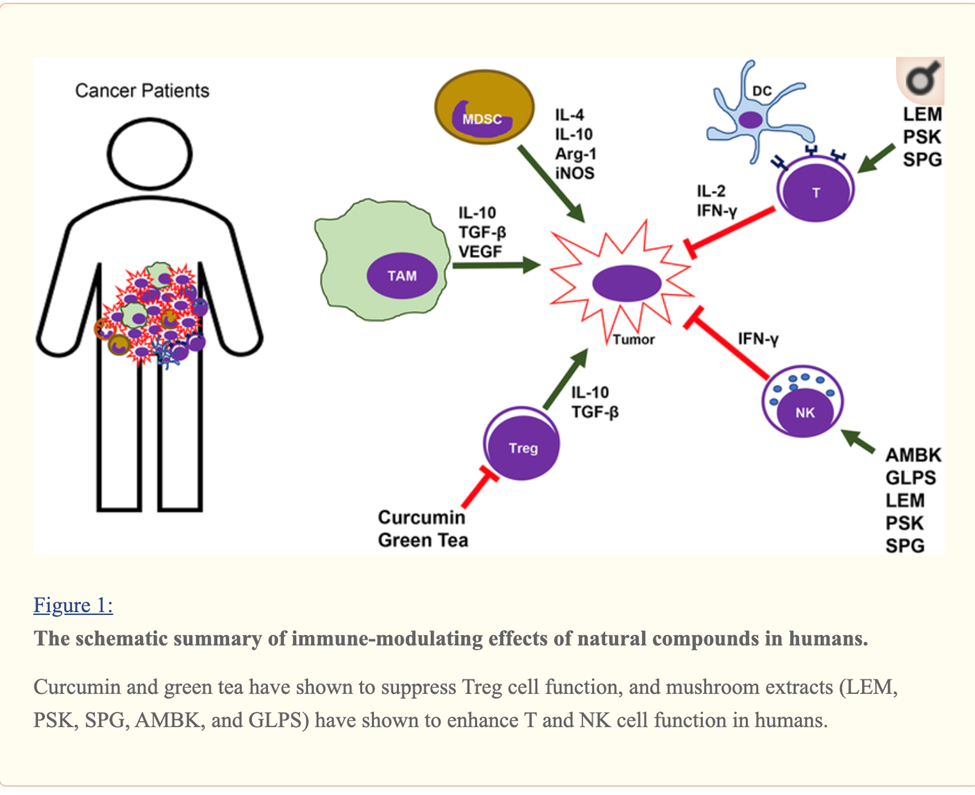
Curcumin, EGCG (Green tea extract) and Resveratrol Immune Enhancing Anti-tumor
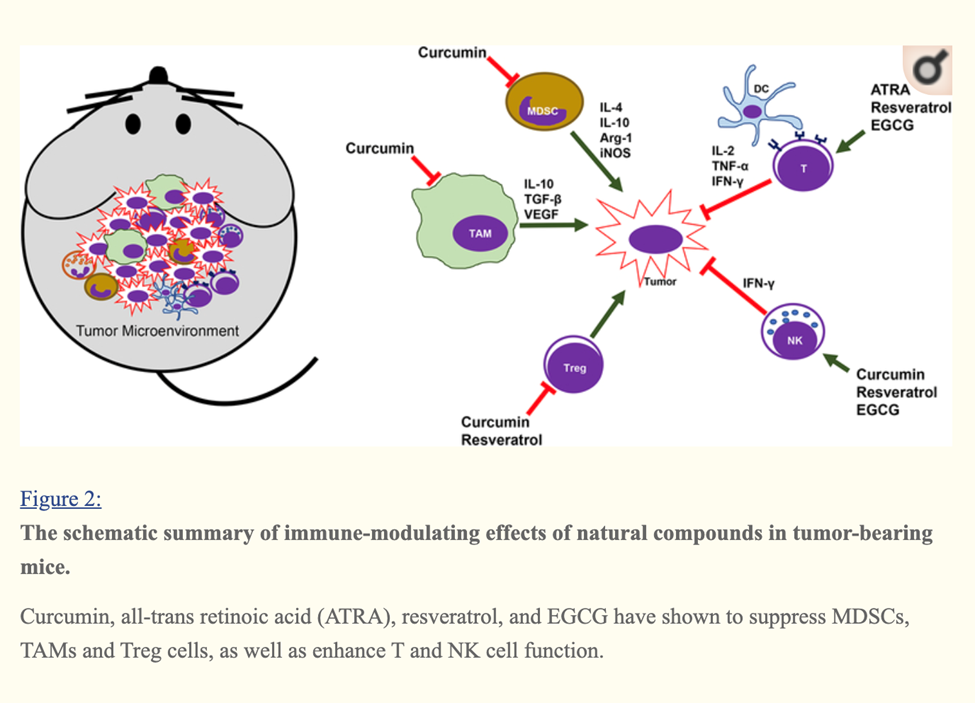
Curcumin, Resveratrol, EGCG and Beta glucan rich mushroom extract have shown promising immune-modulating effects, such as inhibiting myeloid-derived suppressor cells and enhancing natural killer and cytolytic T cells, in tumor-bearing animal models.
Many other studies involving either genetic models or xenograft models of other types of cancer have demonstrated that curcumin can reverse the tumor-favoring microenvironment, leading to further investigations of curcumin combined with standard cancer therapies. One study combined the anti-PD-L1 antibodies with bisdemethoxycurcumin (BDMC), a natural dimethoxy derivative of curcumin, in C57BL/6 mice with metastasized bladder cancer (MB49 cells).
They observed that BDMC alone increased levels of tumor-infiltrating CD8+ T cells, IFN-γ secretion in the blood, and decreased the number of tumor-infiltrating MDSCs.
Importantly, the combination of anti-PD-L1 antibodies with curcumin further enhanced the secretion of IFN-γ, granzyme B, and perforin from CD8+ T cells.[29]
Furthermore, curcumin has been combined with vaccines against tumor development. For instance, one group applied two vaccination strategies to BALB/c mice bearing triple-negative breast tumors (4T1 cells). One strategy gave the mice curcumin and all the vaccinations after tumors had developed. The other treated the mice with curcumin before they were inoculated with tumor cells and then vaccinated the animals after tumors developed. Interestingly, the second strategy was more effective against metastasis than the first, as it decreased the production of pro-inflammatory cancer promoting cytokines (IL-6), and increased levels of anti-tumor cytokines (IL-12 and IFN-γ).[30]
A cocktail of Turmeric- curcumin, Green tea epicatechin gallate and resveratrol—increased levels of tumor-infiltrating NK cells and CD8+ cytolytic T cells in C57BL/6 mice bearing HPV+ mouse lung cancer (TC-1 cells).
The combination formula repolarized tumor promoting M2-like TAMs to tumor fighting M1-like TAMs in the tumors.[31]
Combined with Taxanes
Docetaxel (30 or 75 mg) has been clinically approved and widely used for the treatment of metastatic castration-resistant prostate cancer.[32] However, prolonged treatment with docetaxel can cause severe toxicity in patients. A study found that the combined treatment of docetaxel (10 nM) and curcumin (20 µM) for 48 h significantly inhibited proliferation and induced apoptosis in prostate cancer (PC-3) (DU145 and PC3) cells compared to curcumin and docetaxel alone. The data further demonstrated that curcumin enhances the efficacy of docetaxel in PC-3 cells via modulation of COX-2, p53, NF-κB, phospho-Akt, PI3K, and receptor tyrosine kinase (RTK).[33] This finding implies that combining curcumin with conventional chemotherapy may act as an effective treatment regimen for patients with prostate cancer to reduce cytotoxicity and overcome drug-resistance induced by docetaxel.
Curcumin is a taxane sensitizer for tumors and chemoprotector for normal organs, including in cases of lung cancer.[34]
Combined with Gemcitabine
Curcumin induces apoptosis and inhibits the growth of orthotopic human non-small cell lung cancer xenografts. A research study evaluated the effect of curcumin on the expression of nuclear factor κB-related proteins in vitro and in vivo and on growth and metastasis in an intralung tumor mouse model.
H1975 NSCLC cells were treated with curcumin (0-50μM) alone, or combined with gemcitabine or cisplatin. The effects of curcumin were evaluated in cell cultures and in vivo, using ectopic and orthotopic lung tumor mouse models. Western blot analysis showed that the expressions of IkB, nuclear p65, cyclooxygenase 2 (COX-2) and p-ERK1/2 were down-regulated by curcumin in vitro. Curcumin potentiated the gemcitabine- or cisplatin-mediated antitumor effects. Curcumin reduced COX-2 expression in subcutaneous tumors in vivo and caused a 36% decrease in weight of intralung tumors (P=.048) accompanied by a significant survival rate increase (hazard ratio=2.728, P=.036). Curcumin inhibition of COX-2, p65 expression and ERK1/2 activity in NSCLC cells was associated with decreased survival and increased induction of apoptosis. Curcumin significantly reduced tumor growth of orthotopic human NSCLC xenografts and increased survival of treated athymic mice. Researchers note that to evaluate the role of curcumin in chemoprevention and treatment of NSCLC, further clinical trials are required.[36]
Curcumin Induces PTEN and Improves the Cytotoxicty of Gemzar Against Pancreatic Cancer
Curcumin induces cancer cell growth arrest and apoptosis in vitro, but its poor bioavailability in vivo limits its antitumor efficacy. Researchers noted that in previous evaluations of the bioavailability of novel analogues of curcumin compared with curcumin, they found that the analogue CDF exhibited greater systemic and pancreatic tissue bioavailability.
In a study reported in Cancer Research, scientists evaluated the effects of CDF or curcumin alone or in combination with gemcitabine on cell viability and apoptosis in gemcitabine-sensitive and gemcitabine-resistant pancreatic cancer (PC) cell lines. Mechanistic investigations revealed a significant reduction in cell viability in CDF-treated cells compared with curcumin-treated cells, which were also associated with the induction of apoptosis, and these results were consistent with the downregulation of Akt, cyclooxygenase-2, prostaglandin E(2), vascular endothelial growth factor, and NF-kappaB DNA binding activity.
The researchers documented attenuated expression of miR-200 and increased expression of miR-21 (a signature of tumor aggressiveness) in gemcitabine-resistant cells relative to gemcitabine-sensitive cells. Interestingly, CDF treatment upregulated miR-200 expression and downregulated the expression of miR-21, and the downregulation of miR-21 resulted in the induction of PTEN. These results prompt further interest in CDF as a drug modality to improve treatment outcome of patients diagnosed with PC as a result of its greater bioavailability in pancreatic tissue.[37]
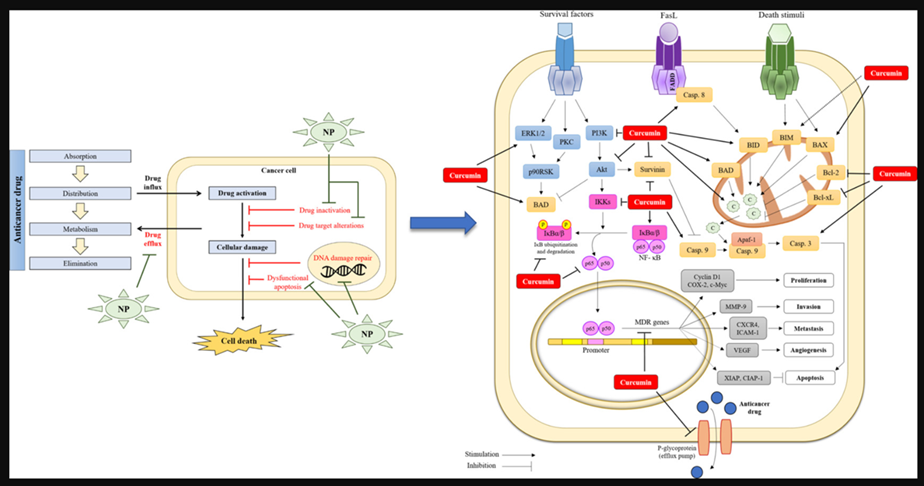
Mechanisms of action of combination curcumin and chemotherapy drugs in vitro and in vivo. Co-treatment with curcumin and chemotherapy drugs such as docetaxel, metformin, 5-fluorouracil, doxorubicin, cisplatin, and celecoxib enhance the synergistic effect via modulating several signaling pathways and thus inhibit cancers such as prostate, hepatocellular, gastric, Hodgkin lymphoma, bladder, and colorectal. Akt: protein kinase B; COX-2: cyclooxygenase-2; EGFR: epidermal growth factor receptor; MMP2/9: matrix metalloproteinase-2/9; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor kappa B; p-ERK1/2: phospho-extracellular signal-regulated kinase 1/2; PI3K: phosphoinositide 3-kinase; p-MEK: phospho-mitogen-activated protein kinase; STAT3: signal transducer and activator of transcription 3; VEGF: vascular endothelial growth factor; VEGFR2: vascular endothelial growth factor receptor 2.
Pathways whereby Curcumin inhibits multi-drug resistance
Combined with Paclitaxel
Paclitaxel (PTX) is a key member of the taxane family with potential anti-tumor activity against different cancers. Notably, PTX has demonstrated excellent proficiency in elimination of cancer in clinical trials. This chemotherapeutic agent is isolated from Taxus brevifolia and is a tricyclic diterpenoid. However, resistance of cancer cells into PTX chemotherapy has endangered its efficacy. In addition, administration of PTX is associated with a number of side effects such as neurotoxicity, hepatotoxicity, and cardiotoxicity, demanding novel strategies in obviating PTX issues.
Curcumin is a pharmacological compound with diverse therapeutic effects including anti-tumor, antioxidant, anti-inflammatory, and anti-diabetic properties. Curcumin appears to enhance the anti-tumor activity of PTX against different cancers. Additionally, curcumin administration reduces the adverse effects of PTX due to its excellent pharmacological activities.[40]
Combined with Metformin
Data from an in vitro study showed that combining metformin (10 mM) and curcumin (5 and 10 µM) can induce apoptosis and inhibit metastasis and invasion in HepG2 and PLC/PRF/5 cells. The anticancer effects could be attributed to the vascular endothelial growth factor (VEGF), MMP2/9, and vascular endothelial growth factor receptor 2 (VEGFR-2) inhibition, PTEN and p53 activation, and epidermal growth factor receptor (EGFR)/STAT3 and NF-κB/mTOR/Akt/PI3K suppression. Data from an in vivo study further showed that co-treatment with metformin and curcumin significantly suppressed hepatocellular carcinoma compared to curcumin (60 mg/kg) and metformin (150 mg/kg) alone in a xenograft mouse model.[41]
Combined with Celecoxib and COX-2 inhibitor
Celecoxib is another selective inhibitor of COX-2, an enzyme induced by different stimuli including inflammation.[42] Celecoxib (75 µM for 16 h) has shown an ability to induce apoptosis and suppress tumor angiogenesis in several types of cancer.[43] However, the long-term use of Celecoxib leads to an adverse outcome such as cardiovascular toxicity.[44] The combination of curcumin and Celecoxib was shown to reduce cancer cell growth in vitro compared to Celecoxib alone. A study reported by Lev-Ari et al. revealed that curcumin (10–15 µmol/L) and physiological dosage of Celecoxib (5 µmol/L) exhibited a synergistic inhibitory effect against human colorectal cancer (HT-29) cells. The study showed that the combination of curcumin and Celecoxib induces apoptosis in HT-29 cells via downregulation of COX-2 expression, suggesting that curcumin synergistically augments the growth inhibitory effects of Celecoxib in human colon cancer cell lines in vitro.[45]
Another anti-cancer property of curcumin seems to be overcoming multidrug resistance.[46] For example, it inhibits P-glycoprotein expression in mouse leukemia L1210/Adr cell lines. Cells treated with a combination of PI3K inhibitor and curcumin displayed lower P-glycoprotein expression in comparison to cells treated only with PI3K inhibitor. It was suggested that curcumin may act by inhibition of the PI3K/Akt/ NF-κB pathway.[47]
Curcumin was found to be a safe and non-toxic dietary supplement. Its efficacy of was studied during clinical trials.[48],[49]
In the mouse model, a high curcumin supplementation (100 mg/kg) was able to improve glucose and insulin intolerance through activating the AMPK pathway, showing the potential involvement of curcumin in metabolism.[50] Modulation of AMPK (AMP-activated protein kinase) improves glucose/insulin efficiency and adds the cancer suppressing effects of curcumin. AMPK acts as a cellular energy sensor, and curcumin selectively can enhance normal cell AMPK signaling, while suppressing it within the cancer cell. [51],[52]
Curcumin in the Modulation of Aging
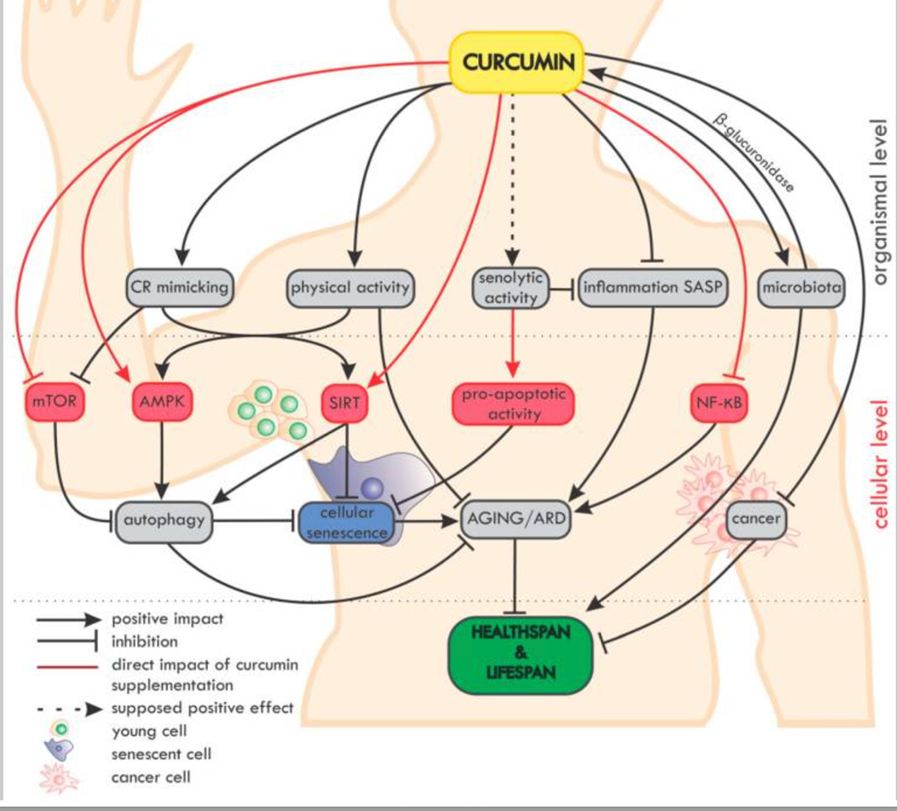
Overview of the impact of curcumin on ageing and age-related diseases (ARD) at the organismal and cellular level. On the organismal level, curcumin mimics caloric restriction (CR) and improves the effectiveness of physical activity (which mimics CR).[53]
In Conclusion
Natural compounds, including curcumin, resveratrol, EGCG, and β-glucan have shown synergistic promising immune-modulating, anti-tumor, and chemo-potentiating effects.
The results of these clinical studies are conclusive, and these studies have established a good foundation for further research focusing on implementing curcumin along with other botanical compounds in clinical oncology. It’s important to note, however, that I never use it as a soloist! I always use a formula that combines curcumin with EGCG, resveratrol, grape seed extract, quercetin, and other botanical extracts. This provides a harmonious approach that best supports healing.
References
[1] Formea CM, Evans CG, Karlix JL. Altered cytochrome p450 metabolism of calcineurin inhibitors: case report and review of the literature. Pharmacotherapy. 2005 Jul;25(7):1021-9. doi: 10.1592/phco.2005.25.7.1021https://pubmed.ncbi.nlm.nih.gov/16006281/. PMID: 16006281.
[2] Zhou SF, Xue CC, Yu XQ, Li C, Wang G. Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Ther Drug Monit. 2007 Dec;29(6):687-710. doi: 10.1097/FTD.0b013e31815c16f5. PMID: 18043468.
[3] Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62(7):919-30. doi: 10.1080/01635581.2010.509835. PMID: 20924967.
[4] Majeed M. The Age of Biotransformation, 2015; Personal communication. Ganjali S, Sahebkar A, Mahdipour E, Jamialahmadi K, Torabi S, Akhlaghi S, Ferns G, Parizadeh SM, Ghayour-Mobarhan M. The Scientific World Journal 2014; doi 10.1155/2014/898361
[5] Liang Shen, Cui-Cui Liu, Chun-Yan An, Hong-Fang Ji, How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies, Scientific Reports volume 6, Article number: 20872 (2016), Published: 18 February 2016
[6] Gordon ON, Luis PB, Sintim HO, Schneider C. Unraveling curcumin degradation: autoxidation proceeds through spiroepoxide and vinylether intermediates en route to the main bicyclopentadione. J Biol Chem. 2015 Feb 20;290(8):4817-4828.
[7] Claus Schneider,* Odaine N. Gordon,1 Rebecca L. Edwards, and Paula B. Luis, Degradation of curcumin: From mechanism to biological implications, J Agric Food Chem. 2015 Sep 9; 63(35): 7606–7614.
[8] He ZY, Shi CB, Wen H, et al. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Invest. 2011;29:208-13.
[9] Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res.2008;14:4491-9.
[10] Shakibaei, M.; Buhrmann, C.; Kraehe, P.; Shayan, P.; Lueders, C.; Goel, A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE 2014, 9, e85397.
[11] Zhou, X.;Wang,W.; Li, P.; Zheng, Z.; Tu, Y.; Zhang, Y.; You, T. Curcumin enhances the e_ects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncol. Res. 2016, 23, 29–34.
[12] Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367.
[13] Vinod, B.S.; Antony, J.; Nair, H.H.; Puliyappadamba, V.T.; Saikia, M.; Narayanan, S.S.; Bevin, A.; Anto, R.J. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013, 4, e505.
[14] Yang H, Huang S, Wei Y, Cao S, Pi C, Feng T, Liang J, Zhao L, Ren G. Curcumin Enhances the Anticancer Effect Of 5-fluorouracil against Gastric Cancer through Down-Regulation of COX-2 and NF- κB Signaling Pathways. J Cancer. 2017 Oct 17;8(18):3697-3706.
[15] Zong, L.; Cheng, G.; Liu, S.; Pi, Z.; Liu, Z.; Song, F. Reversal of multidrug resistance in breast cancer cells by a combination of ursolic acid with doxorubicin. J. Pharm. Biomed. Anal. 2019, 165, 268–275.
[16] Abouzeid, A.H.; Pate, N.R.; Rachman, I.M.; Senn, S.; Torchilin, V.P. Anti-cancer activity of anti-GLUT1 antibody-targeted polymeric micelles co-loaded with curcumin and doxorubicin. J. Drug Target. 2013, 21,994–1000.
[17] Wang, B.L.; Shen, Y.M.; Zhang, Q.W.; Li, Y.L.; Luo, M.; Liu, Z.; Li, Y.; Qian, Z.Y.; Gao, X.; Shi, H.S. Codelivery of curcumin and doxorubicin by MPEG-PCL results in improved e_cacy of systemically administered chemotherapy in mice with lung cancer. Int. J. Nanomed. 2013, 8, 3521–3531.
[18] Duan, J.; Mansour, H.; Zhang,Y.; Deng, X.; Chen,Y.;Wang, J.; Pan,Y.; Zhao, J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2012, 426, 193–201
[19] Guorgui, J.;Wang, R.; Mattheolabakis, G.; Mackenzie, G.G. Curcumin formulated in solid lipid nanoparticles has enhanced e_cacy in Hodgkin’s lymphoma in mice. Arch. Biochem. Biophys. 2018, 648, 12–19..
[20] Ma,W.;Wang, J.; Guo, Q.; Tu, P. Simultaneous determination of doxorubicin and curcumin in rat plasma by LC–MS/MS and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2015, 111, 215–221.
[21]Sadzuka Y, Nagamine M, Toyooka T, Ibuki Y, Sonobe T. Beneficial effects of curcumin on antitumor activity and adverse reactions of doxorubicin, Int J Pharm. 2012 Aug 1;432(1-2):42-9. doi: 10.1016/j.ijpharm.2012.04.062. Epub 2012 Apr 28.
[22] Kaufman, D.S. Challenges in the treatment of bladder cancer. Ann. Oncol. 2006, 17, v106–v112.
[23] Yoon, C.Y.; Park, M.J.; Lee, J.S.; Lee, S.C.; Oh, J.J.; Park, H.; Chung, C.W.; Abdullajanov, M.M.; Jeong, S.J.; Hing, S.K.; et al. The histone deacetylase inhibitor trichostatin A synergistically resensitizes a cisplatin resistant human bladder cancer cell line. J. Urol. 2011, 185, 1102–1111.
[24] Kumar, B.; Yadav, A.; Hideg, K.; Kuppusamy, P.; Teknos, T.N.; Kumar, P. A novel curcumin analog (H-4073) enhances the therapeutic e_cacy of cisplatin treatment in head and neck cancer. PLoS ONE 2014, 9, e93208.
[25] Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22,7265–7279.
[26] Park, B.H.; Lim, J.E.; Jeon, H.G.; Seo, S., II; Lee, H.M.; Choi, H.Y.; Jeon, S.S.; Jeong, B.C. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget 2016, 7, 63870–63886.
[27] Zhu, X.; Shen, H.; Yin, X.; Long, L.; Xie, C.; Liu, Y.; Hui, L.; Lin, X.; Fang, Y.; Cao, Y.; et al. MiR-186 regulation of Twist1 and ovarian cancer sensitivity to cisplatin. Oncogene 2016, 35, 323–332.
[28] Shin SK, Ha TY, McGregor RA, Choi MS. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism, Mol Nutr Food Res. 2011 Dec;55(12):1829-40. doi: 10.1002/mnfr.201100440. Epub 2011 Nov 7.
[29] Shao Y, Zhu W, Da J, Xu M, Wang Y, Zhou J, Wang Z. Bisdemethoxycurcumin in combination with α-PD-L1 antibody boosts immune response against bladder cancer. Onco Targets Ther. 2017 May 22;10:2675-2683. doi: 10.2147/OTT.S130653. PMID: 28579805; PMCID: PMC5449128.
[30] Pan P, Huang YW, Oshima K, et al. The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Crit Rev Food Sci Nutr. 2019;59(6):992-1007. doi:10.1080/10408398.2018.1537237
[31] Mukherjee S, Hussaini R, White R, Atwi D, Fried A, Sampat S, Piao L, Pan Q, and Banerjee P. 2018 TriCurin, a synergistic formulation of curcumin, resveratrol, and epicatechin gallate, repolarizes tumor-associated macrophages and triggers an immune response to cause suppression of HPV+ tumors. Cancer Immunol Immunother.
[32] Tannock, I.F.; deWit, R.; Berry,W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, N.D.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512.
[33] Banerjee, S.; Singh, S.K.; Chowdhury, I.; Lillard, J.W., Jr.; Singh, R. Combinatorial e_ect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer. Front. Biosci. 2017, 9, 235–245.
[34] Yin H., Guo R., Xu Y., et al. Synergistic antitumor efficiency of docetaxel and curcumin against lung cancer. Acta Biochimica et Biophysica Sinica. 2011;44(2):147–153. doi: 10.1093/abbs/gmr106
[35] Goel A, Aggarwal BB. Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer. 2010;62(7):919-30. doi: 10.1080/01635581.2010.509835. PMID: 20924967.
[36] Lev-Ari S, Starr A, Katzburg S, Berkovich L, Rimmon A, Ben-Yosef R, Vexler A, Ron I, Earon G. Curcumin induces apoptosis and inhibits growth of orthotopic human non-small cell lung cancer xenografts. J Nutr Biochem. 2014 Apr 13. pii: S0955-2863(14)00077-1.
[37] Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010 May 1;70(9):3606-17. Epub 2010 Apr 13.
[38] Tan BL, Norhaizan ME. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules. 2019 Jul 10;24(14):2527. doi: 10.3390/molecules24142527. PMID: 31295906; PMCID: PMC6680685.
[39] Ahmad RS, Hussain MB, Sultan MT, et al. Biochemistry, Safety, Pharmacological Activities, and Clinical Applications of Turmeric: A Mechanistic Review. Evid Based Complement Alternat Med. 2020;2020:7656919. Published 2020 May 10. doi:10.1155/2020/7656919
[40] Ashrafizadeh M, Zarrabi A, Hashemi F, Moghadam ER, Hashemi F, Entezari M, Hushmandi K, Mohammadinejad R, Najafi M. Curcumin in cancer therapy: A novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020 Sep 1;256:117984. doi: 10.1016/j.lfs.2020.117984. Epub 2020 Jun 25. PMID: 32593707.
[41] Zhang, H.-H.; Zhang, Y.; Cheng, Y.-N.; Gong, F.-L.; Cao, Z.-Q.; Yu, L.-G.; Guo, X.-L. Metformin in combination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol. Carcinog. 2018, 57, 44–56.
[42] Kim J., Hong S.-W., Kim S., Kim D., Hur D.Y., Jin D.H., Kim B., Kim Y.S. Cyclooxygenase-2 expression is induced by celecoxib treatment in lung cancer cells and is transferred to neighbor cells via exosomes. Int. J. Oncol. 2018;52:613–620.
[43] Kim, J.; Hong, S.-W.; Kim, S.; Kim, D.; Hur, D.Y.; Jin, D.H.; Kim, B.; Kim, Y.S. Cyclooxygenase-2 expression is induced by celecoxib treatment in lung cancer cells and is transferred to neighbor cells via exosomes. Int. J. Oncol. 2018, 52, 613–620.
[44] Solomon, S.D.; McMurray, J.J.; Pfe_er, M.A.; Wittes, J.; Fowler, R.; Finn, P.; Anderson, W.F.; Zauber, A.; Hawk, E.; Bertagnolli, M.; et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005, 352, 1071–1080.
[45] Lev-Ari, S.; Strier, L.; Kazanov, D.; Madar-Shapiro, L.; Dvory-Sobol, H.; Pinchuk, I.; Marian, B.; Lichtenberg, D.; Arber, N. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin. Cancer Res. 2005, 11, 6738–6744.
[46] Rashmi R., Kumar S., Karunagaran D. Human colon cancer cells lacking Bax resist curcumin-induced apoptosis and Bax requirement is dispensable with ectopic expression of Smac or downregulation of Bcl-XL. Carcinogenesis. 2005;26:713–723. doi: 10.1093/carcin/bgi025.
[47] Choi B.H., Kim C.G., Lim Y., Shin S.Y., Lee Y.H. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett. 2008;259:111–118. doi: 10.1016/j.canlet.2007.10.003.
[48] Lao C.D., Ruffin M.T., Normolle D., Heath D.D., Murray S.I., Bailey J.M., Boggs M.E., Crowell J., Rock C.L., Brenner D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006;6:10. doi: 10.1186/1472-6882-6-10.
[49] Sharma R.A., Euden S.A., Platton S.L., Cooke D.N., Shafayat A., Hewitt H.R., Marczylo T.H., Morgan B., Hemingway D., Plummer S.M. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744.
[50] Kubczak, Małgorzata et al. “Molecular Targets of Natural Compounds with Anti-Cancer Properties.” International journal of molecular sciences vol. 22,24 13659. 20 Dec. 2021, doi:10.3390/ijms222413659
[51] Yu, Hua et al. “Curcumin Regulates the Progression of Colorectal Cancer via LncRNA NBR2/AMPK Pathway.” Technology in cancer research & treatment vol. 18 (2019): 1533033819870781. doi:10.1177/1533033819870781
[52] Campbell NK, Fitzgerald HK, Fletcher JM, Dunne A. Plant-Derived Polyphenols Modulate Human Dendritic Cell Metabolism and Immune Function via AMPK-Dependent Induction of Heme Oxygenase-1. Front Immunol. 2019 Mar 1;10:345. doi: 10.3389/fimmu.2019.00345. PMID: 30881359; PMCID: PMC6405514.
[53] Bielak-Zmijewska, Anna et al. “The Role of Curcumin in the Modulation of Ageing.” International journal of molecular sciences vol. 20,5 1239. 12 Mar. 2019, doi:10.3390/ijms20051239