By Donnie Yance
Part 1: Infections, Fevers, and the Immune-Cancer Connection
A surprising discovery shows that a COVID-19 infection can activate immune cells that fight cancer, opening doors to potential new treatments for patients who have run out of options.
One of cancer’s deadliest traits is its ability to hide from our immune system. Unlike other diseases, cancer cells can suppress or trick our body’s natural defenses. They become invisible to immune cells that normally would attack them, and can even manipulate the immune system to help cancer grow while blocking the cells meant to destroy them; these include the important defenders called Cytotoxic T-cells and Natural Killer Cells.
This is what makes the COVID-19 discovery so significant. Scientists studying mice and blood samples from COVID patients found that the virus triggers special immune cells called I-NCMs. These cells have a unique ability to reach places where tumors grow – something most other immune cells cannot do – and they seem to overcome cancer’s usual immune-evading tactics.
The research team discovered that these I-NCMs are more effective at stopping cancer spread than similar, naturally occurring immune cells. Even more promising, the same immune response triggered by COVID can be activated by an approved drug, potentially offering a new way to treat cancer.
“We saw positive results against melanoma, lung, breast, and colon cancer,” said Dr. Ankit Bharat, who led the team that made this discovery. He explained that these special immune cells work in two ways: they can patrol blood vessels and penetrate tumor sites. Once inside the tumor, they signal the body’s natural killer cells to attack the cancer, effectively breaking through cancer’s defensive barriers.
While the research is still in early stages with animal testing, Dr. Bharat believes this approach could help patients with advanced cancers who haven’t responded to other treatments, particularly those whose cancers have become experts at evading the immune system.1
Over the past almost four decades of working with cancer, I have found a relationship between acute illness with high fevers and spontaneous remissions in advanced aggressive cancer, most of which have been pediatric cancers.
Also, there have been several reports and publications on this as well. Accumulating evidence exists for (1) an inverse correlation between the incidence of infectious diseases and cancer risk
and (2) a correlation between infections with fevers and cancer remissions.2
Factors that contribute to spontaneous regression (SR)
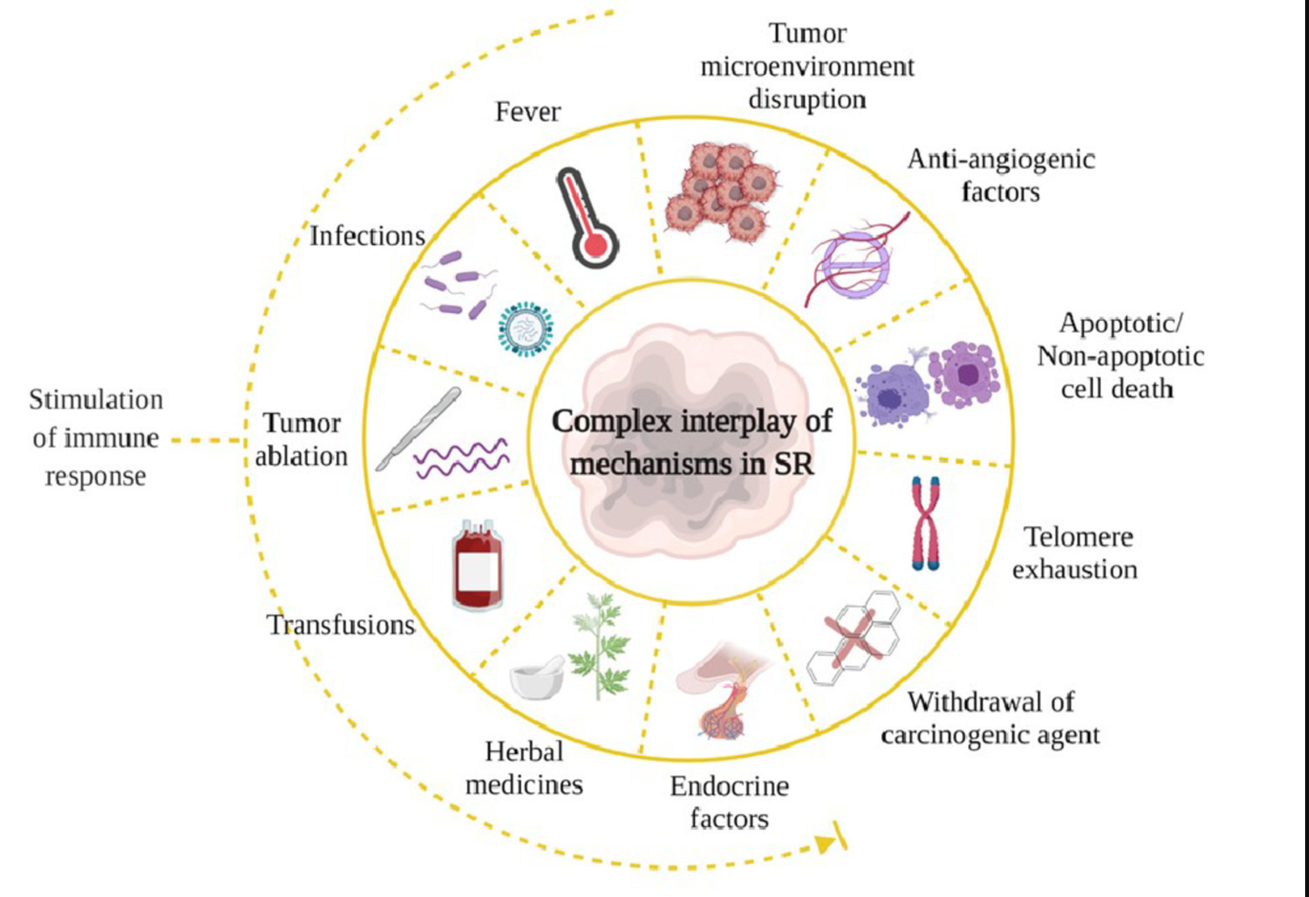
Spontaneous regression (SR) of cancer, while rare, has provided crucial insights into the immune system’s natural capacity to combat malignancies. These remarkable cases, where tumors disappear without conventional treatment, have often been associated with concurrent infections or inflammatory conditions, highlighting the immune system’s central role in tumor control. Historical documentation of SR cases, particularly those coinciding with acute infections, fever, or inflammatory episodes, laid the groundwork for understanding immune-mediated tumor suppression.
Several key patterns emerged from studying spontaneous regression cases:
- Acute infections often preceded or accompanied tumor regression
- Fever appeared to be a crucial component of the regression process
- The immune response generated against pathogens showed cross-reactivity with tumor cells
- Inflammation-induced cytokine cascades created an anti-tumor microenvironment
These observations directly influenced the development of various immunotherapeutic strategies:
- Cancer vaccines designed to stimulate specific anti-tumor immune responses
- Cytokine-based therapies that mimic the immune response to an infection
- Oncolytic viral therapies that selectively infect and destroy cancer cells while stimulating immune responses
- Immune checkpoint inhibitors that remove barriers to natural anti-tumor immunity.3
Coley’s Toxin- the 1st Successful Infectious Immune Activating Treatment For Cancer
The stimulation of an acute infection to create anti-tumor immune responses first gained scientific recognition with the development of Coley’s Toxin. In 1891, Dr. William Bradley Coley, an American-born surgeon and cancer researcher, formulated this groundbreaking treatment using heat-inactivated bacteria (Streptococcus pyogenes and Serratia marcescens). His initial success at New York’s Memorial Hospital, where he successfully treated a sarcoma patient, led to widespread implementation of the therapy.4
The treatment showed remarkable efficacy, even in advanced-stage cancers. Coley identified critical parameters for optimal therapeutic outcomes: the infection needed to induce fever with significantly elevated body temperature.5 The protocol required daily or alternate-day injections directly into the tumor for one to two months, with gradually escalating doses. To prevent relapse, patients required weekly maintenance injections for six months. This regimen proved particularly effective against sarcomas, lymphomas, melanomas, and myelomas.
Despite its therapeutic success, Coley’s Toxin was eventually superseded by radiation therapy and chemotherapy. Several factors contributed to its decline: incomplete understanding of its mechanism of action, concerns about deliberately exposing patients to bacterial products, and variable treatment outcomes. However, this pioneering work illuminated the crucial role of immune system activation in cancer control, a concept later supported by prominent scientists including Paul Ehrlich.6 The last documented success of Coley’s Toxin occurred in China in 1980, where a terminal liver cancer patient received 68 injections over 34 weeks.7
Coley’s innovative approach laid the foundation for modern cancer immunotherapy, earning him recognition as the father of this field. His work demonstrated that strategic immune system activation through controlled infection could mobilize the body’s natural defenses against cancer, a principle that continues to influence contemporary immunotherapy approaches.8,9
Modern analysis of SR cases continues to reveal new mechanisms of immune-mediated tumor control. For instance, molecular studies of SR cases have identified specific immune cell populations and signaling pathways that become activated during successful tumor elimination. This understanding has led to the development of more targeted immunotherapeutic approaches, including engineered T-cell therapies and personalized cancer vaccines.
The study of SR cases thus represents a crucial bridge between historical observations and contemporary immunotherapy, demonstrating how the body’s natural defense mechanisms can be harnessed for therapeutic benefit. This knowledge continues to inform the development of novel immunotherapeutic strategies, making SR research an invaluable component of cancer immunology.10
The Gut Microbiota On Cancer Immunotherapy
Recent studies have revealed the profound influence of gut microbiota on cancer immunotherapy outcomes, highlighting a crucial microbiome-immunity-cancer axis. Specifically, certain bacterial families have emerged as key modulators of immune checkpoint inhibitor efficacy. Members of the Bifidobacteriaceae family enhance patient responses to anti-PD-L1 (anti-programmed cell death protein ligand-1) therapy through multiple mechanisms: they stimulate dendritic cell maturation, enhance CD8+ T cell priming, and promote interferon-γ production in the tumor microenvironment.11,12, 13 Similarly, bacteria from the Bacteroidaceae family significantly improve responses to anti-CTLA-4 (anti-cytotoxic T-lymphocyte antigen 4) therapy by augmenting T cell responses and modulating mucosal immunity.14
The mechanisms underlying this microbiome-mediated enhancement involve several pathways:
- Beneficial bacteria produce short-chain fatty acids and secondary bile acids that regulate T-cell differentiation and function.
- They maintain intestinal barrier integrity, preventing harmful inflammation
- They promote beneficial immune responses
This bacterial modulation can significantly impact treatment outcomes, with studies showing up to a three-fold improvement in progression-free survival in patients harboring favorable gut microbiota compositions.
These findings have important clinical implications, suggesting that microbiome modulation through targeted probiotics, prebiotics, or fecal microbiota transplantation could potentially optimize immunotherapy responses. Moreover, microbiome profiling could serve as a predictive biomarker for immunotherapy efficacy, enabling more personalized treatment approaches in cancer therapy.
Several reports indicate that Akkermansia muciniphila affects glucose metabolism, lipid metabolism, and intestinal immunity, and that certain food ingredients such as polyphenols may increase the abundance of Akkermansia muciniphila in the gut.15
Akkermansia is a Gram-negative anaerobic (thrives in low-oxygen environments) bacteria that lives in the mucus layer of the colon. It is one of the most abundant bacteria in the gut microbiome, constituting up to 5% of the total bacteria in the gut in healthy adults.
- Akkermansia & Gut Mucus: In the mucus layer, it feasts on the glycoproteins (i.e. proteins decorated with sugar molecules) that make up this mucus, and creates the short chain fatty acids acetate and propionate as byproducts.
- Repairing Leaky gut: Mucus breakdown by Akkermansia stimulates the differentiation of stem cells into mucus-producing cells. Therefore, the net effect of Akkermansia is improvement in rates of mucus production and the integrity of the mucus layer.
Improving The Effectiveness Of Cancer Immunotherapy
Akkermansia muciniphila (A. muciniphila) can significantly inhibit carcinogenesis and improve anti-tumor effects, thus increasing the effectiveness of cancer immunotherapy and decreasing the likelihood of side effects. A. muciniphila mediates effects on host metabolism and can influence the efficacy of anti-PD-1-based immunotherapy against cancer. The mechanisms for these effects remain poorly understood, but both appear to be immune mediated and correlate with type 1 immunity.
In particular, responses to anti-PD-1-based immunotherapy in humans correlated with interferon gamma production by peripheral T cells when incubated with A. muciniphila antigens in vitro. The potential clinical applications of A. muciniphila on cancer immunotherapy are also proposed, which have great prospects for anti-tumor therapy.16,17
Improvements In Gastric-Cancer Treatment
The gut microbiome is pivotal in tumor occurrence and development, and there is a close relationship between Akkermansia muciniphila and cancer immunotherapy. One study evaluated the effects of this bacteria on gastric cancer cells.
Proteins from the membranes of Akkermansia were found to support apoptosis (self-destruction) of the cancer cells and exerted an immune-stimulating effect (by promoting M1 polarization of macrophages, enhancing expression of cytotoxic T-lymphocyte-related cytokines and suppressing that of Treg-related cytokines).
Additionally, Akkermansia can inhibit gastric tumor growth and enhance the infiltration of immune cells into tumor tissues.18
Akkermansia populations are also supported by key prebiotics. Prebiotics are molecules that pass through our digestive systems intact and reach to the colon where they can support the growth of beneficial microbes. The prebiotics that promote Akkermansia growth include polyphenols and human milk oligosaccharides (HMOs).19

Polyphenols
Polyphenols are antioxidant molecules found in a variety of foods, and can often be identified by their vibrant colors. For example, the dark purple-blue pigments found in blueberries, blackberries, cranberries, and red cabbage are a group of polyphenols known as anthocyanins.
Polyphenols have the ability to neutralize free radicals and, in doing so, protect against oxidative stress and inflammation.
Polyphenols derived from grapes act to increase the abundance of Akkermansia in the intestinal tract; as a result, they have been shown to enhance intestinal barrier function and incretin secretion from intestinal endocrine cells.20,21 Polyphenols derived from cranberries have also been reported to increase the abundance of Akkermansia, as well as help suppress obesity, insulin resistance, and intestinal inflammation.22
In addition to their direct anti-inflammatory effects, polyphenols also feed Akkermansia and another important group of bacteria in the gut known as Bifidobacteria.
Therefore, polyphenol consumption can support the colonization of the gut by these two key groups of bacteria.
- Grape polyphenols: Research has demonstrated that the consumption of grape polyphenols increases the levels of Akkermansia present in the gut. Moreover, this increase in Akkermansia was associated with enhanced intestinal barrier integrity and elevated secretion of hormones known as incretins from small intestinal cells. Incretins play an important role in the maintenance of healthy weight, glucose levels, and satiety.23
- Cranberry polyphenols: Cranberry polyphenols have also been shown to support Akkermansia populations and exert protective effects against obesity, insulin resistance, and inflammation within the intestines.24,25
- Apple peel polyphenols: Similarly, animal models suggest that the polyphenols present in red apple peels, known as procyanidins, partially protect against the weight gain, inflammation, and liver dysfunction characteristic of a high fat, high sugar diet. These benefits were attributed to improvements in the microbiome, one of which was heightened levels of Akkermansia.
Probiotics and Postbiotics
In addition to dietary and lifestyle strategies, research efforts are also geared towards determining whether Akkermansia probiotics could confer benefits to human health. Although there is a current lack of research examining the effects of Akkermansia probiotics in humans, several studies have been conducted in animal models of diet-induced obesity.
Rodent models of diet-induced obesity (DIO) fed a high fat, high sugar diet mimicking a Standard American Diet exhibit suppressed populations of Akkermansia and develop similar conditions as humans with diet-induced obesity including systemic inflammation, arterial plaque, breakdown of the gut mucus layer, and insulin resistance. Upon 4-8 weeks of treatment with an Akkermansia probiotic, DIO mice exhibit restored levels of Akkermansia as measured in fecal samples, with no other changes to the microbiome composition.
Additionally, these animals showed: reduced levels of inflammation, shrinking of plaques, restored gut mucus layer thickness, reduced body weight, and improved insulin sensitivity. However, these benefits were only seen with the higher dose probiotic not the lower dose suggesting that dosing may be an important consideration when using Akkermansia probiotics in the setting of obesity and metabolic dysfunction in humans.
Bifidobacteria Cross-feeds Akkermansia
In other animal studies, researchers supplemented obese rodents with a probiotic containing Bifidobacterium animalis (*subspecies lactis) to see if raising the levels of this species in the gut could indirectly boost levels of Akkermansia.
Indeed, treatment with this species for 14 weeks resulted in a dramatic increase in the fecal content of A. muciniphila. The group treated with this probiotic also gained less weight than control animals also fed the high fat, high sugar diet.26
The mechanism by which B. animalis promotes the growth of A. muciniphila is still unknown, however the researchers postulate that it may be due to enhanced butyrate production facilitated by B. animalis, as butyrate stimulates mucus production in the gut.
Conclusion
The unexpected link between COVID-19 infections and anti-tumor immune responses has opened new avenues for cancer research and treatment. By triggering specialized immune cells like I-NCMs, COVID-19 demonstrates how acute infections can sometimes awaken the body’s natural defenses against cancer. This phenomenon mirrors historical observations, such as those from Coley’s Toxin, where infections and fever played pivotal roles in spontaneous cancer regressions. These findings reaffirm the immune system’s incredible potential to combat malignancies when properly activated.
Bifidobacteria and Akkermansia have emerged as critical players in enhancing cancer immunotherapy. Their ability to promote immune responses, support apoptosis in cancer cells, and improve the effectiveness of treatments like anti-PD-1 therapy highlights the importance of microbiome health in cancer care. Dietary interventions with polyphenols, prebiotics, and targeted probiotics show promise in optimizing the gut to support these beneficial bacteria.
As we continue to unravel the complex relationship between infections, immune responses, and the microbiome, it becomes clear that harnessing these natural mechanisms could revolutionize cancer treatment. Future therapies may combine traditional approaches, with strategies designed to activate the immune system, and optimize microbiome health, offering new hope for patients with difficult-to-treat cancers.
In Part 2 of this article, we will take a deep dive into herbal medicine and all that it has to offer for immune enhancement and cancer care.
About the Author:
Donald R. Yance is the founder of the Mederi Center. A Clinical Master Herbalist and Certified Nutritionist, Donnie is renowned for his extraordinary knowledge and deep understanding of the healing properties of plants and nutrition, as well as of epigenetics, laboratory medicine, oncologic pathology, and molecular oncology. He is a professional member of the American Herbalists Guild, National Association of Nutrition Professionals, Academy of Integrative Health and Medicine, and the Society for Integrative Oncology.
References:
1. Xianpeng Liu, G.R. Scott Budinger, Ankit Bharat, Inducible CCR2 + nonclassical monocytes mediate the regression of cancer metastasis, Published November 15, 2024, J Clin Invest. 2024;134(22):e179527. https://doi.org/10.1172/JCI179527.
2. Kleefa Wayne B. Jonasb Wolfgang Knoglerc Werner Stenzingerd, Fever, Cancer Incidence and Spontaneous Remissions Ralf, Neuroimmunomodulation 2001;9:55–64
3. Gudapureddy Radha, Manu Lopus, The spontaneous remission of cancer: Current insights and therapeutic significance, Translational Oncology, Volume 14, Issue 9, 2021,101166, ISSN 1936-5233,
4. S.A. Hoption Cann, J.P. Van Netten, C. Van Netten, Dr William Coley and tumour regression: a place in history or in the future, Postgrad. Med. J. 79 (938) (2003) 672–680.
5. W.B. Coley, Contribution to the knowledge of sarcoma, Ann. Surg. 14 (3) (1891) 199–220.
6. Dobosz, T. Dzieciątkowski, The intriguing history of cancer immunotherapy, Front Immunol. 10 (2019) 2965.
7. P. Kucerova, M. Cervinkova, Spontaneous regression of tumour and the role of microbial infection-possibilities for cancer treatment, Anticancer Drugs 27 (4)(2016) 269–277.
8. J.A. Thomas, M. Badini, The role of innate immunity in spontaneous regression ofcancer, Indian J. Cancer 48 (2) (2011) 246–251.
9. Gudapureddy Radha, Manu Lopus, The spontaneous remission of cancer: Current insights and therapeutic significance, Translational Oncology, Volume 14, Issue 9, 2021,101166, ISSN 1936-5233,
10. G. Lizee, W.W. Overwijk, L. Radvanyi, J. Gao, P. Sharma, P. Hwu, Harnessing the power of the immune system to target cancer, Annu. Rev. Med. 64 (2013) 71–90.
11. Z. Dai, J. Zhang, Q. Wu, et al., Intestinal microbiota: a new force in cancer immunotherapy, Cell Commun. Signal 18 (1) (2020) 90. Jun 10.
12. V. Gopalakrishnan, C.N. Spencer, L. Nezi, et al., Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients, Science 359 (6371) (2018) 97–103. Jan 5.
13. B. Routy, E. Le Chatelier, L. Derosa, et al., Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors, Science 359 (6371) (2018) 91–97. Jan 5.
14. Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Bérard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015 Nov 27;350(6264):1079-84. doi: 10.1126/science.aad1329. Epub 2015 Nov 5. PMID: 26541610; PMCID: PMC4721659
15. Naito Y, Uchiyama K, Takagi T. A next-generation beneficial microbe: Akkermansia muciniphila. J Clin Biochem Nutr. 2018 Jul;63(1):33-35. doi: 10.3164/jcbn.18-57. Epub 2018 Jun 20. PMID: 30087541; PMCID: PMC6064808
16. Fan S, Jiang Z, Zhang Z, Xing J, Wang D, Tang D. Akkermansia muciniphila: a potential booster to improve the effectiveness of cancer immunotherapy. J Cancer Res Clin Oncol. 2023 Nov;149(14):13477-13494. doi: 10.1007/s00432-023-05199-8. Epub 2023 Jul 25. PMID: 37491636.
17. Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, Brown EM, Graham DB, Xavier RJ, Moon JJ, Barton GM. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019 Jun 21;364(6446):1179-1184. doi: 10.1126/science.aaw7479. PMID: 31221858; PMCID: PMC6645389.
18. Fang J, Zhang H, Zhang X, Lu X, Liu J, Li H, Huang J. Akkermansia muciniphila improves gastric cancer treatment by modulating the immune microenvironment. Future Microbiol. 2024;19(6):481-494. doi: 10.2217/fmb-2023-0210. Epub 2024 Apr 17. PMID: 38629914; PMCID: PMC11216265.
20. Roopchand DE, Carmody RN, Kuhn P, et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes. 2015;64:2847–2858. doi: 10.2337/db14-1916
21. Reunanen J, Kainulainen V, Huuskonen L, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81:3655–3662. doi: 10.1128/AEM.04050-14.
22. Anhê FF, Marette A. A microbial protein that alleviates metabolic syndrome. Nat Med. 2017;23:11–12. doi: 10.1038/nm.4261.
23. Anhê FF, Pilon G, Roy D, Desjardins Y, Levy E, Marette A. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? Gut Microbes. 2016;7(2):146-53. doi: 10.1080/19490976.2016.1142036. Erratum in: doi: 10.1136/gutjnl-2014-307142. PMID: 26900906; PMCID: PMC4856456.
24. Pierre JF, Heneghan AF, Feliciano RP, Shanmuganayagam D, Roenneburg DA, Krueger CG, Reed JD, Kudsk KA. Cranberry proanthocyanidins improve the gut mucous layer morphology and function in mice receiving elemental enteral nutrition. JPEN J Parenter Enter Nutr 2013; 37:401-9; PMID:23064255; http://dx.doi.org/ 10.1177/0148607112463076
25. Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, Marette A. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015 Jun;64(6):872-83. doi: 10.1136/gutjnl-2014-307142. Epub 2014 Jul 30. PMID: 25080446.
26. Xiao, M., Zhang, C., Duan, H. et al. Cross-feeding of bifidobacteria promotes intestinal homeostasis: a lifelong perspective on the host health. npj Biofilms Microbiomes 10, 47 (2024). https://doi.org/10.1038/s41522-024-00524-6